ABSTRACT
Case description: A 67-year-old man was admitted with progressive heart failure due to blood culture-negative endocarditis of the aortic valve. Urgent aortic valve replacement was needed. Polymerase chain reaction (PCR) testing of samples of the explanted aortic valve revealed Tropheryma whipplei. The patient received ceftriaxone, followed by long-term co-trimoxazole. Recent arthralgia may have been a diagnostic clue.
Conclusions: Whipple’s endocarditis should be considered in patients with arthralgia and blood culture-negative endocarditis (BCNIE).
LEARNING POINTS
- Whipple’s endocarditis should be considered in patients with symptoms of arthralgia and blood culture-negative endocarditis (BCNIE).
- Serum polymerase chain reaction is the main diagnostic test.
- Both physician awareness and multidisciplinary management by regional endocarditis teams are recommended strategies to provide optimal patient care.
KEYWORDS
Whipple’s endocarditis, Tropheryma whipplei, blood culture-negative endocarditis
CASE DESCRIPTION
A 67-year-old Caucasian man with a history of ischaemic stroke, mild aortic valve insufficiency, anaemia and in recent months arthralgia, was admitted to the cardiac care unit with severe progressive dyspnoea, orthopnoea, and peripheral oedema resistant to oral diuretics. His regular medications consisted of amlodipine, pantoprazole, acetylsalicylic, levothyroxine and furosemide. Cardiac symptoms had been present for several months for which he had visited the outpatient cardiology clinic.
Methods and procedures
Transthoracic echocardiography (TTE) showed moderate aortic valve stenosis, and mild mitral and tricuspid valve regurgitation, but no signs of vegetation, abscess or valve destruction. Laboratory analyses showed a C-reactive protein 13 mg/l, white blood cell count 8.60×109/l, and NT-proBNP 260 pmol/l. Blood cultures were negative. A PET-CT scan was also negative. Treatment with oral diuretics was started.
On physical examination at hospital admission, blood pressure was 124/58 mmHg, respiratory rate was 31 per minute, oxygen saturation was 100% without supplementation, and temperature was 36.4°C. Bilateral crackles were heard in the basal lung fields and pitting oedema was seen in the extremities. Cardiac auscultation was hampered due to loud respiratory sounds, drowning out any (new) cardiac murmur.
The electrocardiogram showed sinus tachycardia (105 beats per minute), a few supraventricular premature beats, left atrial enlargement and left ventricular hypertrophy. Blood results revealed a normocytic anaemia, haemoglobin 6.9 mmol/l, C-reactive protein (CRP) 19 mg/l, white blood cell count 9.86×109/l, NT-proBNP 810 pmol/l and creatinine 140 µmol/l. Chest x-ray showed bilateral pleural fluid, particularly right-sided, and a normal cardiac silhouette.
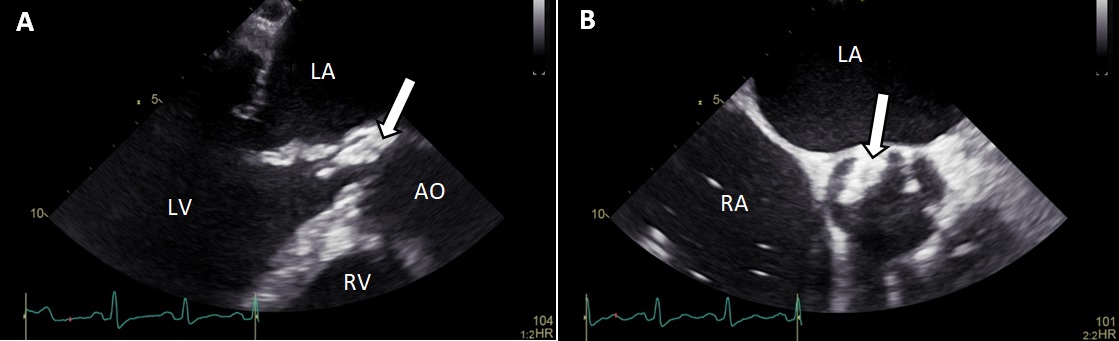
Intravenous diuretics were started, but without response. Transoesophageal echocardiography (TOE) revealed mild mitral valve regurgitation, and severe aortic valve insufficiency with mobile vegetation at the non-coronary cusp (size 28×8 mm; Fig. 1). Endocarditis treatment with intravenous administration of amoxicillin (12 g/24 h) and ceftriaxone (4 g/24 h) was started. Blood cultures remained negative. In addition, serology for Bartonella henselae, Coxiella burnetii, Mycoplasma pneumoniae, cytomegalovirus, Legionella pneumoniae, Influenza A and Coxsackie virus remained negative. The patient’s clinical condition deteriorated despite 1 week of treatment. He also suffered from impaired memory and cognition, which made additional history taking inadequate.
Figure 1. Transoesophageal aortic long-axis view (A) and short-axis view (B) with a large vegetation (arrowhead) at the non-coronary cusp.
AO, aorta; LA, left atrium; LV, left ventricle; RA, right atrium; RV, right ventricle
He was transferred to a tertiary hospital for urgent cardiac surgery. Open heart surgery revealed a severely damaged aortic valve which was replaced with a 23 mm bioprosthesis. An abscess was seen in the intraventricular septum close to the right coronary cusp, from which necrotic material was surgically removed. The central area of the anterior mitral valve leaflet appeared perforated. It was reconstructed using a bovine pericardial patch and a 34 mm annular prosthesis. Annuloplasty was used to repair the insufficient tricuspid valve (De Vega technique). Immediate post-operative TOE showed the aortic valve prosthesis was performing well and no residual mitral or tricuspid regurgitation.
A polymerase chain reaction test (PCR) for 16S ribosomal DNA, followed by nucleic acid sequencing on two different samples of the explanted aortic valve tissue, revealed Tropheryma whipplei as the causative agent. This was confirmed by a T. whipplei-specific PCR performed by a reference laboratory. Therapy was continued with ceftriaxone 2 g a day for 4 weeks, followed by oral co-trimoxazole 960 mg twice daily for 12 months. Three weeks after cardiac surgery, the patient had recovered well and was discharged home. On cardiopulmonary exercise testing, maximal work was 82 Watt (64% of the predicted value) and the VO2 maximal value was also low at 18.5 ml/min/kg. Spirometry was normal. Thereafter, the patient started his physical rehabilitation program. Three months post-operatively, he was free of complaints. TTE showed normal left and right ventricular function with mild residual aortic valve regurgitation. Treatment with diuretics was discontinued.
DISCUSSION
Whipple’s endocarditis is a rare infective condition [1]. The incidence rate has been estimated at between 1 and 6 new cases per 10,000,000 people per year worldwide [2]. It can be accompanied by other symptoms of Whipple’s disease, although 20% of cases are isolated endocarditis[3]. Classic Whipple’s disease presents with several symptoms including articular involvement, heart failure, weight loss, gastro-intestinal symptoms and neurological symptoms [1, 4]. Arthralgia, arthritis and/or spondylodiscitis are seen in the majority of patients with classic Whipple’s disease, who often present with unexplained polyarthritis. This frequently results in a misdiagnosis of Whipple’s disease as inflammatory rheumatoid disease [4]. Patients with endocarditis due to T. whipplei do not present with the classic features of endocarditis. As demonstrated in our patient, the clinical presentation often does not include fever, peripheral stigmata or inflammatory response and hence does not meet the Duke criteria for endocarditis [1].
The main diagnostic methods for detecting T. whipplei are histopathology and PCR [1, 4]. PCR is generally performed on heart valve tissue to diagnose Whipple’s endocarditis. Blood may be tested by PCR, but sensitivity is low although the positive predictive value is high [4]. The cultivation of T. whipplei is difficult, and its presence would not be detected by routine blood and tissue culture [1, 4]. The European Society of Cardiology (ESC) guidelines explicitly recommend serological testing for C. burnetii, T. whipplei, fungi (Candida and Aspergillus species), and Bartonella, Legionella, Brucella and Mycoplasma species in patients who are suspected of having BCNIE [2].
The eradication of T. whipplei requires prolonged antibiotic treatment [4]. Recommended antibiotic treatment, based on small observational studies, consists of an initial phase with intravenous ceftriaxone or penicillin, followed by a prolonged maintenance phase with cotrimoxazole and sulfamethoxazole for at least 12 months, or alternatively, doxycycline with or without hydroxychloroquine [1,3,5].
Conclusion
Whipple’s endocarditis should be considered in patients with symptoms of arthralgia and BCNIE. The clinical presentation is variable, and patients rarely fulfil the Duke criteria for the diagnosis of endocarditis. Arthralgia should be considered as a diagnostic clue. Patients with Whipple’s endocarditis need long-term antibiotic therapy, and relapse and mortality rates remain high. Both physician awareness and multidisciplinary management by regional endocarditis teams are recommended strategies.