ABSTRACT
The coexistence of systemic lupus erythematosus (SLE) and Crohn’s disease (CD) is very rare. The usual sequence of occurrence is CD followed by SLE, where CD treatment with anti-tumour necrosis factor (anti-TNF) induces the latter. Here, we present a case of this rare combination but with sequence reversal. The patient was unresponsive to steroids and we achieved remission with infliximab.
LEARNING POINTS
- Crohn’s disease complicating stable systemic lupus erythematosus is extremely rare.
- Although it may delay time to diagnosis, it is important to rule out other common causes such as infections and medication-induced colitis.
- If the patient is steroid unresponsive, infliximab might be a reasonable therapeutic alternative.
KEYWORDS
Systemic lupus erythematosus, infliximab, Crohn's disease
INTRODUCTION
Systemic lupus erythematosus (SLE) is a multisystem immune disorder which can involve any organ. Crohn’s disease (CD) is an inflammatory bowel disease (IBD) with some extra-intestinal manifestations. Both diseases share gastrointestinal symptomology and patients with either disease may present with abdominal pain, diarrhoea or signs of intestinal obstruction. The coexistence of these two diseases is very rare and in such cases, CD usually precedes SLE as treatment of CD with anti-tumour necrosis factor (anti-TNF) can induce SLE. Reversed sequence of disease occurrence is exceptionally rare, and no proposed triggers have been identified [1]. Here we present the case of a 34-year-old man with SLE and subsequent CD colitis.
CASE DESCRIPTION
A 34-year-old man was diagnosed with SLE at 30 years of age. The diagnosis was based on fatigue, generalized body swelling, and joint pain involving the metacarpophalangeal and proximal interphalangeal joints. There was no joint swelling but he had a history of morning stiffness lasting for more than 30 minutes. There was facial puffiness and lower limb oedema. Moreover, the patient was anaemic with a haemoglobin level of 7.2 g/dl. A direct agglutination test was positive. Antinuclear antibodies, anti-dsDNA antibodies and anti-Smith antibodies were positive with high titres. C3 and C4 complement levels were low and 24-hour urine protein was 5.9 g. A kidney biopsy was performed and mycophenolate mofetil (MMF) 3 g/day was initiated. The biopsy showed class IV and V lupus nephritis, and tacrolimus 4 mg/day was added. The patient was in remission on MMF and tacrolimus for 4 years and his latest 24-hour urine protein in January 2020 was 270 mg.
In July 2020, the patient presented to our emergency department with a 5-day history of abdominal pain and bloody diarrhoea. The pain was aching in nature and over the entire abdomen without peritoneal signs. On examination, he was afebrile with tachycardia of 111 beats/minute and blood pressure was 100/70 mmHg. The abdomen was tender without organomegaly. Per rectal examination was positive for fresh blood but did not demonstrate any local causes of haematochezia. His haemoglobin was 4.1 g/dl with normal MCV and MCH. He was resuscitated with 3 units of packed RBCs and upper and lower endoscopies were performed the next morning.
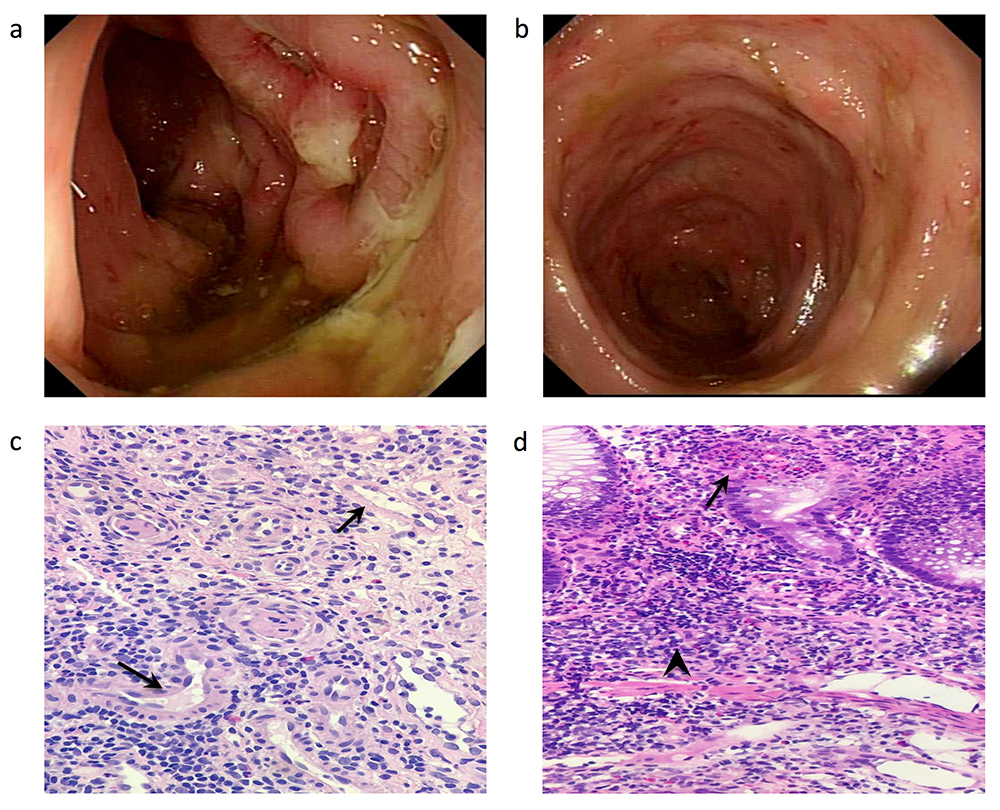
The upper endoscopy was unremarkable, but the colonoscopy showed multiple ulcers extending from the rectum to the ileocaecal valve. The largest ulcer was 15 mm in diameter with stigmata of recent bleeding (Fig. 1a,b). The ileum contained ulcers with no bleeding and both ileal and colonic biopsies were taken. The biopsies demonstrated active colitis and focal ulceration with ileal inflammation and lymphoid aggregates with no signs of viral cytopathic changes, vasculitis or dysplasia (Fig. 1c,d). Clostridium difficile and other infections including mycobacterial infections were ruled out. A high dose of 60 mg prednisolone was initiated but no significant improvement was noticed. Since the patient was on an adequate dose of immunosuppressant medications including steroids and striking pathological features were lacking at the time, both IBD and SLE flare were less likely. Medication-induced colitis (tacrolimus and MMF) was assumed by consensus and the medications. Medication-induced colitis (tacrolimus and MMF) was assumed by consensus and the medications were withheld. The patient was discharged with clinic follow-up.
Figure 1. Endoscopic and histopathological features of the patient at presentation. (a) Sigmoid colon showing a large ulcer with stigmata of recent bleeding. (b) Descending colon with multiple small ulcers. (c) Blood vessels (arrows) with no signs of vasculitis. (d) Colon with cryptitis (arrow) and deep lymphoid aggregates (arrowhead)
Despite the cessation of GI bleeding, the patient persistently complained of moderate abdominal pain and frequent bowel motions, which did not completely resolve despite 3 months of discontinuance of the possible offending medications. After 3 months, the patient returned to the emergency department with per rectal bleeding. After stabilization and preparation, the patient underwent colonoscopy.
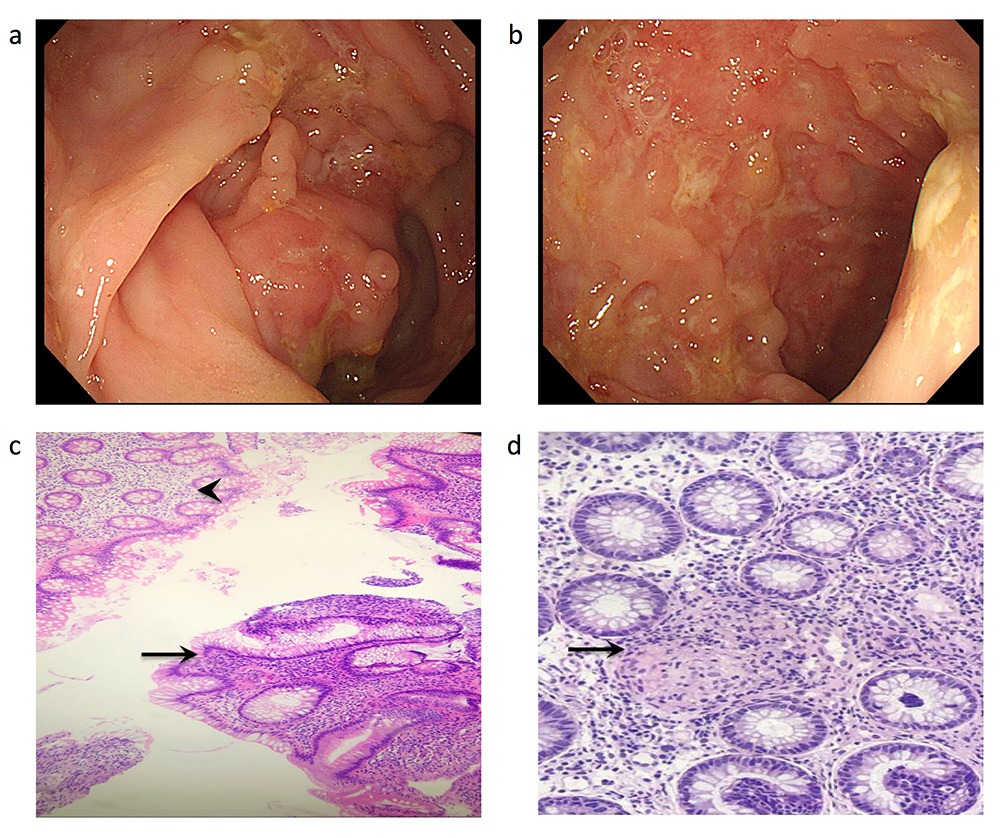
Ulcers in the ascending, transverse and descending colon ranging from 5 to 20 mm in diameter and involving more than 30% of the colon surface were seen (Fig. 2a,b). Biopsies from the involved area showed moderate active colitis, cryptitis and crypt abscesses with granulomas (Fig. 2c,d). Based on the endoscopic and pathological findings, the patient was diagnosed with CD. Three months after the initiation of infliximab and azathioprine, both diseases were in remission.
Figure 2. Endoscopic and histopathological features of the patient 3 months after medication discontinuation. (a) Descending colon showing skip lesions and cobblestoning. (b) Ascending colon with a cobblestone appearance. (c) Skip lesions on colon biopsy with normal mucosa (arrowhead) opposite active chronic colitis (arrow). (d) Colon biopsy showing non-caseating granuloma (arrow)
DISCUSSION
Although this patient was on adequate doses of immune modulators for SLE (MMF and tacrolimus), they did not suppress or change the natural history of CD. After infections and SLE-related GI manifestations such as vasculitis are excluded, cessation of medication is a reasonable approach. Regardless of order of occurrence of the two diseases, anti-TNF for CD is a cause of drug-induced lupus and MMF and tacrolimus are a cause of drug-induced colitis [1, 2]. While this approach will delay the diagnosis for weeks to months as in this case, it is unavoidable. The span of time medication-induced colitis takes to resolve and the time required to develop the pathognomonic features of CD seen on endoscopy and histopathology are major determinants.
Since the year 2000, only six cases with the same disease sequence have been reported [1, 3–7]. In three of the reported cases, the patient was started on infliximab with subsequent remission of both conditions [3–5]. The remaining three cases received a steroid-based regimen, which was ineffective in our patient. Although using infliximab as a therapeutic choice might induce SLE flare, the three cases reported good outcomes. Nevertheless, extended long-term follow-up is lacking. Ustekinumab is an alternative for anti-TNF in CD but lack of efficacy in SLE and discontinuation of the phase III LOTUS trial render it useless. Finally, JAK inhibitors are a reasonable second-line therapy that is effective in both disorders [8].
The diagnosis of SLE or CD is challenging. The coexistence of these two diseases is very rare and diagnosing such a concurrence requires a careful per exclusionem approach. If CD complicates SLE in steroid unresponsive patients, infliximab may be a reasonable choice of therapy. Long-term follow-up regarding its effectiveness is needed.