ABSTRACT
Ciliocytophthoria is a phenomenon where degenerated cells in infections or malignancy can present as ciliated cells on microscopy and so may be confused with ciliated parasitic infection. We present an interesting case of recurrent shortness of breath, misdiagnosed as chronic obstructive pulmonary disease exacerbations leading to unnecessary exposure to antimicrobials and steroids. The case was diagnosed as Strongyloides hyper-infection syndrome. Another finding worth mentioning was that ciliated cells noted on broncho-alveolar lavage were thought to be a co-infection with Balantidium colibut were later confirmed as ciliocytophthoria.
LEARNING POINTS
- Strongyloides hyper-infection syndrome should be considered in the differential diagnosis of a patient from an endemic area presenting with non-resolving respiratory symptoms.
- Ciliocytophthoria is a type of degenerative process where degenerated cells can appear ciliated on microscopy.
- Balantidium coliappears to be similar but is much larger and has cilia circumferentially compared with ciliocytophthoria which has a polar distribution of cilia.
KEYWORDS
Strongyloidiasis, hyperinfection syndrome, ciliocytophthoria
CASE DESCRIPTION
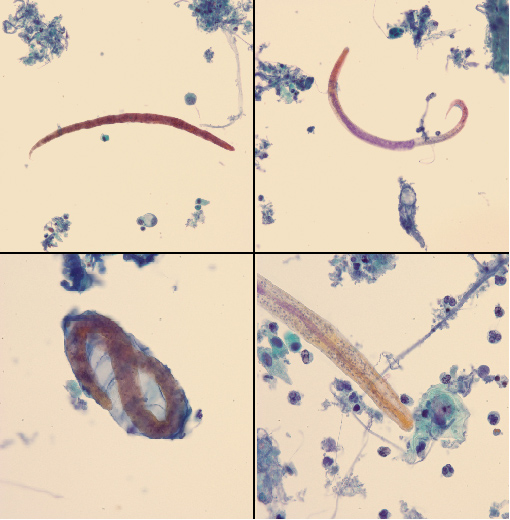
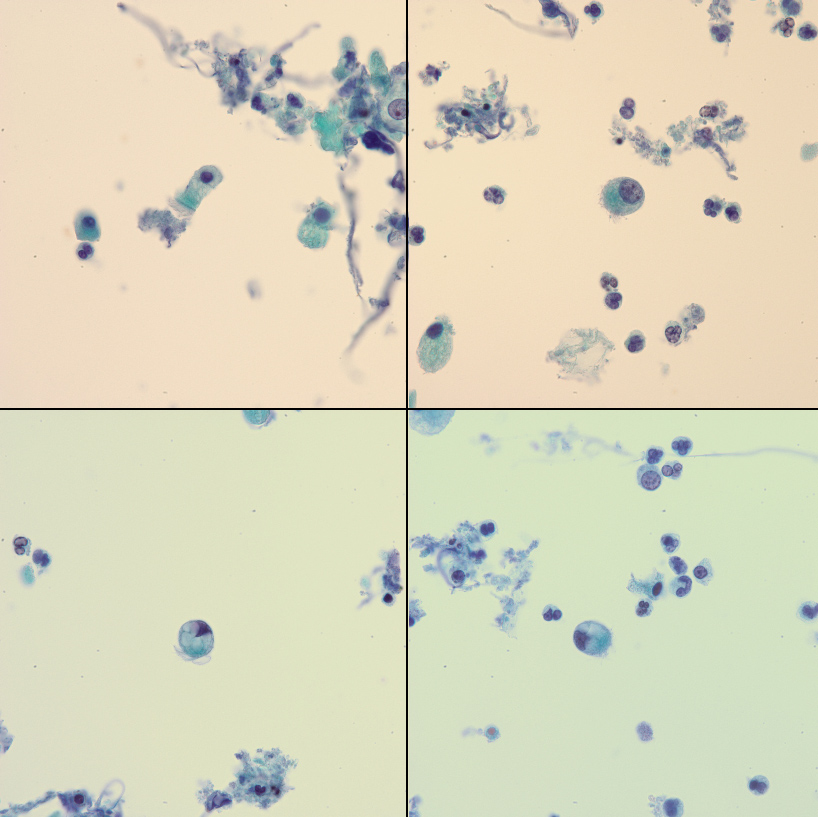
A 68-year-old woman with stable chronic lymphocytic leukaemia (CLL), hypogammaglobulinemia, chronic obstructive pulmonary disease (COPD) and hypertension had multiple admissions to a local hospital for presumed COPD exacerbations. She had received multiple courses of steroids and antibiotics over the previous 7 months. She was transferred to a tertiary care facility secondary to respiratory failure requiring ventilatory support. She did not have any known exposure to pigs or other farm animals. She did not have eosinophilia on initial presentation. A Papanicolaou (PAP) stain of the broncho-alveolar lavage (BAL) sample obtained at admission showed rhabditiform larvae of Strongyloides stercoralis (Fig. 1, Video 1) and a single ciliated respiratory epithelial cell (Fig. 2, Video 2) which was initially identified as Balantidium coli. The patient’s stool specimens were also positive for Strongyloides.
Figure 1. Pap smear of bronchoalveolar lavage specimen 1. Top right and left (200×) show rhabditiform larvae of Strongyloides stercoralis. Bottom left (500×) shows larva to be enclosed in ovum remnant. Bottom right (500×) shows a close-up view of the larva
Video 1. Wet preparation of bronchoalveolar lavage specimen 1. This shows an actively moving Strongyloides stercoralis larva
Figure 2. Pap smear of bronchoalveolar lavage specimen 2. Top left (1000×) shows a single ciliated respiratory epithelial cell. Compare this with ciliocytophthoria (CCP) shown in the top right, bottom right and bottom left panels (1000×). Note the small size and polar cilia
Video 2. Wet preparation of bronchoalveolar lavage specimen 2. This shows ciliocytophthoria (CCP). Note clumping of cells with polar cilia
Due to her history of hypogammaglobulinemia, the patient was treated with intravenous immunoglobulins (IVIG) in addition to a 14-day course of ivermectin (200 µg/kg daily) and albendazole after Strongyloides was seen in BAL and the stool sample. She also received doxycycline and metronidazole for the presumed B. coliinfection.
However, the BAL sample was re-evaluated and the diagnosis of B. coliwas later revised in favour of ciliocytophthoria as B. coliwas never isolated from stool specimens. The hospital course was further complicated by vancomycin-resistant Enterococcus faecium (VRE) bacteraemia, Pseudomonas aeruginosa ventilator-associated pneumonia and relapsing enterococcal (non-VRE) meningitis. The patient died after a prolonged hospital stay.
DISCUSSION
Strongyloidiasis is caused by the nematode Strongyloides stercoralis. It is endemic in tropical and subtropical areas. In the USA, the Appalachian region has the highest rates of infection [1]. The filariform larvae penetrate the skin and migrate through the lungs to the gastrointestinal tract to develop into adult worms. Autoinfection allows persistence of this nematode for several decades in asymptomatic individuals. The cycle of autoinfection can lead to a hyper-infection syndrome in immunocompromised patients. Clinical findings in the hyper-infection syndrome may be attributable to the direct consequences of organ invasion by the filariform larvae and lead to secondary bacteraemia, pneumonia or even meningitis due to bloodstream seeding originating from gastrointestinal tract transmigration. Eosinophilia may be absent. The likelihood of developing the hyper-infection syndrome is increased if cell-mediated immunity is impaired by underlying malignancy, malnutrition, alcoholism, haemopoietic stem cell transplantation, hypogammaglobulinemia or the administration of corticosteroids or cytotoxic drugs [2]. Management involves treating the underlying immune deficiency state and use of ivermectin with or without albendazole.
Ciliocytophthoria (CCP) refers to a type of degenerative process observed in the ciliated cells of bronchial epithelium, often associated with viral infections [3], tonsillitis [4] and malignancy [5]. Joseph Leidy described the phenomenon in respiratory samples of asthmatic patients in the 19th century. Hilding observed aberrant nasal structures mimicking parasitic cells in 1930 [6], while Papanicolaou coined the term ciliocytophthoria in 1956 [5]. It has been reported from respiratory [3], gynaecological [7] and peritoneal washings specimens [8] and is seen on fixed, stained cytological specimens as well as fresh preparations. It is often confused with the only known ciliated parasite of humans, B. coli. CCP can be differentiated from B. coliinfection on the basis of size, distribution of cilia and presence of a nucleus [9]. B. coliis a parasite of the large intestine, often found in areas of close human contact with pigs in the background of poor sanitary conditions. The organism is much larger and possesses cilia circumferentially compared with CCP which have a polar distribution of cilia. B. colioften have a kidney-shaped, indented macronucleus, while CCP may be anucleate. High-magnification microscopy and special stains may be used to confirm the diagnosis of CCP.
The existence of CCP as a degenerative process has been corroborated in several in vitro studies using electron microscopy. Recent case reports of isolation of and infection by uncommon protozoa in humans [10] and knowledge of multiflagellated protozoa of the order Hypermastigida inhabiting the alimentary canals of certain insects has raised several questions regarding dismissal of all CCP as a purely degenerative phenomenon.