ABSTRACT
Hypoplastic coronary artery disease is a rare congenital anomaly that may present with ischaemic heart disease, heart failure or sudden cardiac death (SCD). We describe a case of cardiac arrest in a healthy young man. Work-up revealed a hypoplastic left anterior descending artery. The patient underwent cardioverter-defibrillator implantation for secondary prevention.
LEARNING POINTS
- Hypoplastic coronary artery disease (HCAD) is a rare cause of cardiac arrest and should be suspected in cases of sudden cardiac death (SCD) in young adults.
- The mechanism in HCAD leading to ventricular fibrillation cardiac arrest is not well understood.
- Implantable cardioverter-defibrillator (ICD) implantation is recommended for secondary prevention of ventricular fibrillation.
KEYWORDS
Hypoplastic coronary artery disease, cardiac arrest
CASE DESCRIPTION
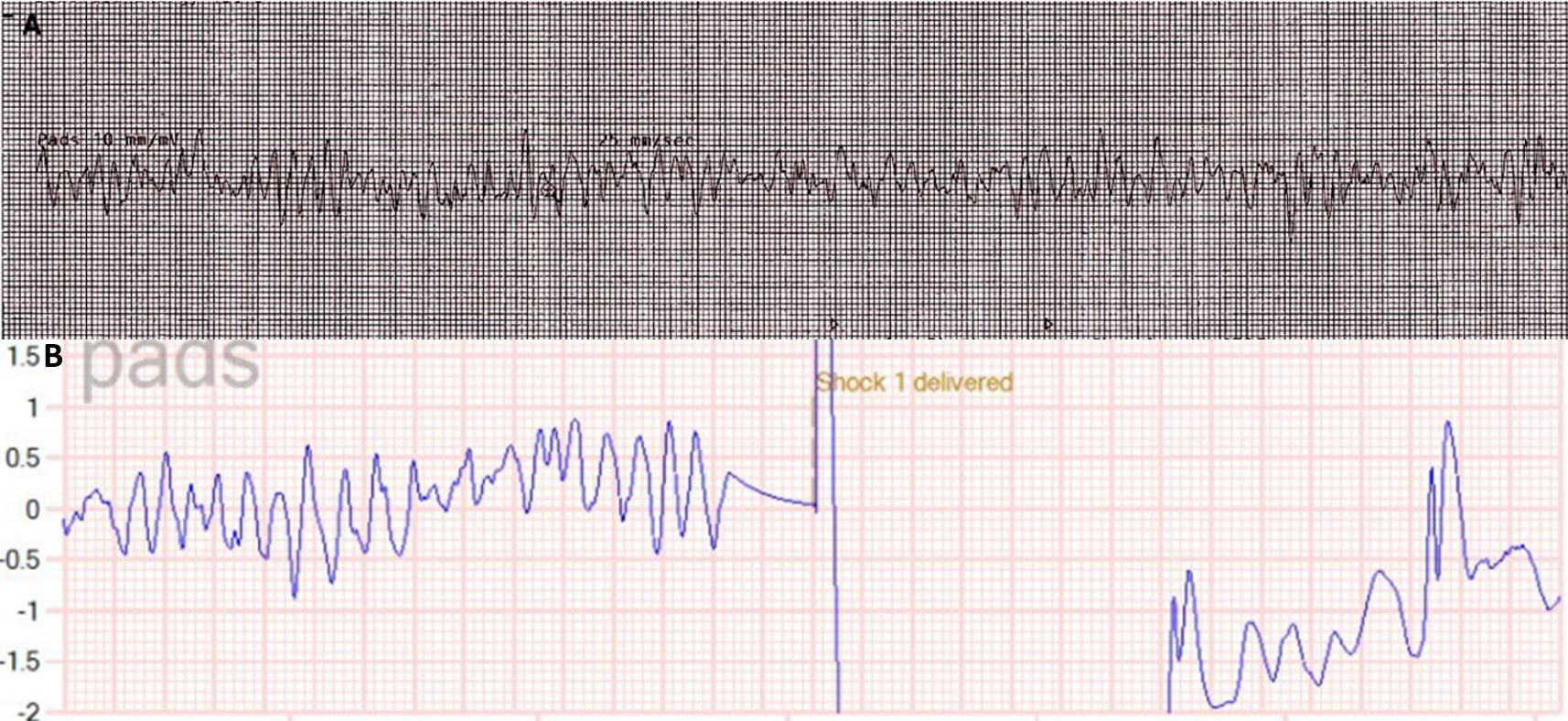
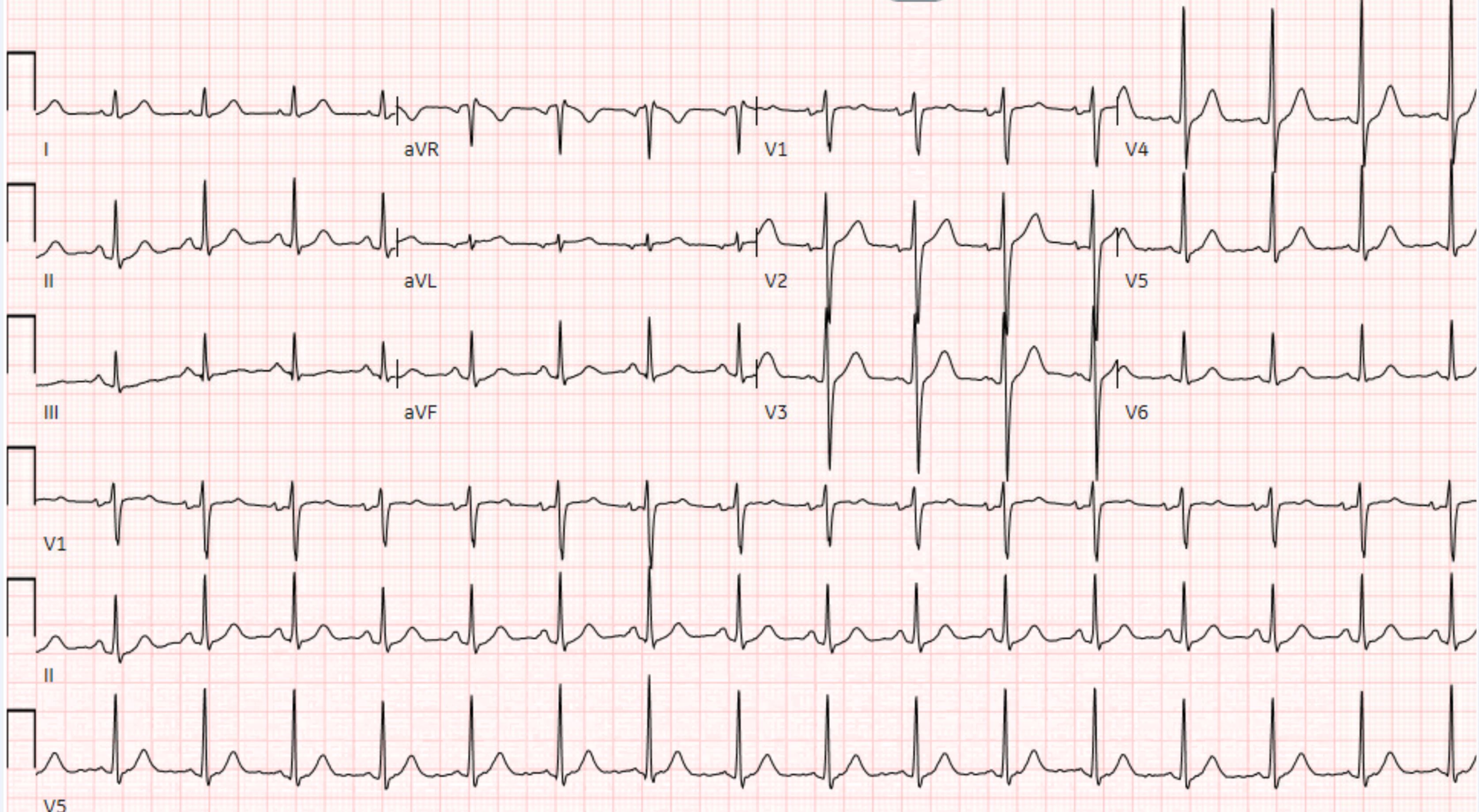
A 26-year-old male former cross-country runner presented with ventricular fibrillation cardiac arrest occurring while running. He was defibrillated with immediate return of spontaneous circulation. Figure 1 shows electrocardiogram (ECG) tracings before (Fig, 1A) and after defibrillation (Fig. 1B). There were no prodromal symptoms including light-headedness, chest pain or shortness of breath. He denied any previous history of syncope or any exertional symptoms, particularly while running. He reported no over-the-counter or prescribed medication use. Physical examination was notable for a thin, healthy-appearing male, in no distress. The patient was afebrile with a blood pressure of 121/64 mmHg, respiratory rate of 20 breaths per minute, and oxygen saturation of 97% on room air. The cardiopulmonary exam was within normal limits with no appreciable jugular venous distension or lower extremity oedema noted. The ECG demonstrated normal sinus rhythm with a rate of 97 beats per minute with normal PR, QRS and QTc intervals, and without stigmata of any arrhythmogenic syndromes or pathological Q waves (Fig. 2). The patient had a history of childhood asthma. His family history was unremarkable and there was no history of sudden cardiac arrest (SCA). He denied alcohol consumption, a history of smoking or illicit drug use.
Figure 1. Electrocardiogram tracings before and after defibrillation: (A) pre-resuscitation rhythm strip showing ventricular fibrillation; (B) post-defibrillation rhythm strip showing return of normal sinus rhythm
Figure 2. Post-resuscitation ECG
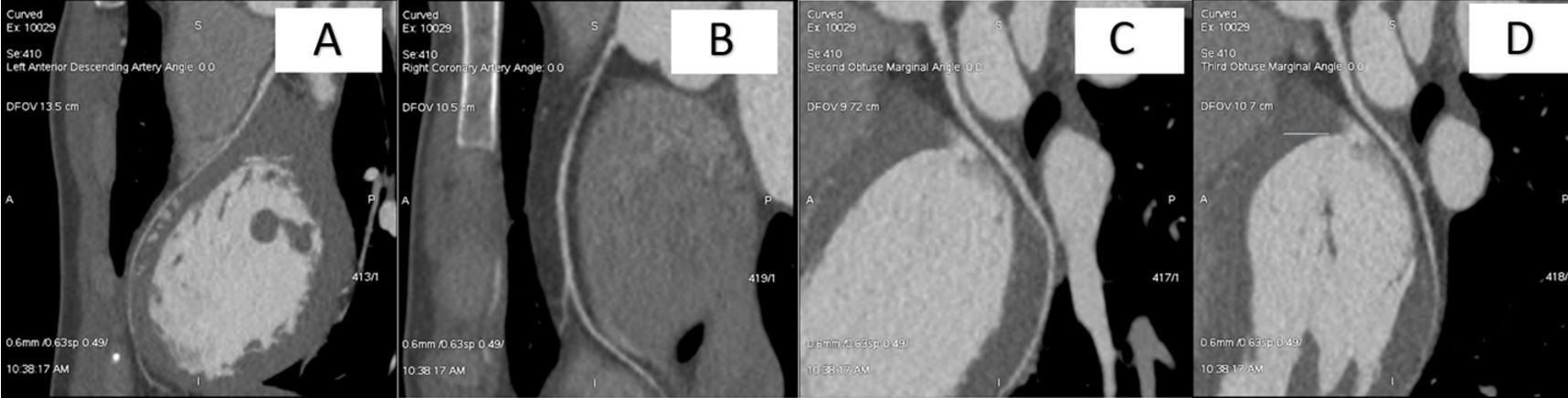
Troponin peaked at 0.24 ng/ml (normal range 0–0.4 ng/ml). Other laboratory tests including electrolytes, complete blood count and Lyme titres, were normal. The patient had a transthoracic echocardiogram (TTE) demonstrating an ejection fraction of 67% with normal wall thickness and no structural abnormalities. Cardiac magnetic resonance imaging (CMR) showed a right ventricle of normal size and wall thickness. CMR imaging with gadolinium enhancement was negative for infiltrative disease or myocardial scars. Coronary computed tomography angiography (CCTA) showed a hypoplastic left anterior descending (LAD) artery, measuring 2.4 mm proximally, 1.4 mm at mid-level, and 1.2 mm distally (Fig. 3A), without collateral branches. The right coronary artery (RCA) was noted to be 2.4 mm in diameter (Fig. 3B). The left circumflex artery (LCX) was noted to be 2.8 mm (Fig. 3C,D). There was no evidence of coronary atherosclerosis or anomalous coronary anatomy. The patient underwent outpatient genetic testing and an exercise stress test, which were both negative.
Valvular and structural causes of SCD were ruled out with TTE and CMR. Arrhythmogenic causes including arrhythmogenic right ventricular cardiomyopathy (ARVC) and catecholaminergic polymorphic ventricular tachycardia (CPVT) were ruled out given the unremarkable CMR and the negative exercise stress test, respectively. The unusually small LAD artery was consistent with hypoplastic coronary artery disease (HCAD). Given the lack of other identifiable causes of cardiac arrest, it was felt the most likely cause of cardiac arrest was myocardial ischaemia in the territory of the LAD artery leading to ventricular fibrillation.
We did consider coronary microvascular disease in our differential diagnosis but felt this was much less likely given the patient was young and otherwise healthy.
Figure 3. Coronary computed tomography angiography of the major coronary arteries: (A) left anterior descending (LAD) artery; (B) right coronary artery (RCA); (C) obtuse marginal 2 (OM2) of the left circumflex artery (LCX); (D) obtuse marginal 3 (OM3) of the left circumflex artery (LCX)
The decision was made to forgo confirmatory testing with myocardial perfusion imaging (MPI) as it would not have changed our management. Moreover, from a cost and patient radiation exposure perspective, we did not feel it prudent to pursue additional testing in an otherwise healthy male.
The patient was diagnosed with sudden cardiac death (SCD) due to myocardial ischaemia. A subcutaneous implantable cardioverter-defibrillator (ICD) for secondary prevention was implanted and the patient was discharged home without medication. The patient has remained without recurrence of arrhythmias or symptoms for 12 months. He has returned to a normal quality of life following instructions to limit strenuous exercise.
DISCUSSION
First reported in 1970, HCAD is a rare congenital anomaly characterized by a truncated or smaller diameter major coronary artery or one of its branches [1, 2]. Although more commonly found in isolation, HCAD may also be seen with other congenital cardiac abnormalities including left ventricular hypoplasia and anomalous origin of the left coronary artery [1, 3].
While the incidence of coronary artery anomalies in the general population is 1%, the incidence of HCAD is unknown [1, 4]. Of the fewer than 30 cases of HCAD reported in the literature, the RCA and LCX are most often involved, with only nine cases reported involving the LAD artery [1, 2, 5]. There is one documented case of HCAD involving all three major coronary arteries.
Presenting symptoms include chest pain, palpitations, dyspnoea, syncope, myocardial infarction and SCD. The majority of HCAD cases reported in the literature have presented as SCD in the young adult and paediatric population [1, 3, 5]. Cases of isolated myocardial infarction in an 11-year-old boy and a 20-year-old man have been reported [2, 3] as well as one case of haemorrhagic myocardial infarction in a 16-year-old athlete [6].
It is hypothesized that SCD from HCAD results from arrhythmia triggered by myocardial ischaemia. However, the mechanism by which HCAD causes myocardial ischaemia is not well understood [4]. Inability to provide adequate coronary flow during exercise, coronary artery vasospasm, and watershed perfusion deficits have all been proposed as the mechanism of myocardial ischaemia in patients with HCAD [3,4,7]. However, inconsistent reproducibility of ischaemia related to coronary anomalies poses a challenge in better elucidating this mechanism. Given an otherwise extensive negative work-up, we felt confident that our patient’s SCD was due to exercise-induced ischaemia. Pursuing confirmatory testing with MPI would not have changed our overall management and would have subjected our patient to not only radiation exposure, but also an increased cost associated with his hospital stay. This case exemplifies cost-effective medical practices.
ICD placement is recommended for secondary prevention of SCD based on professional society guidelines [3, 4, 7]. There are no documented cases of ICD placement for primary prevention of SCD in patients with HCAD [4]. Genetic testing can be of value in determining causes of SCD as it may have implications regarding testing relatives and children of the gene carrier [8]. Our patient had genetic testing performed to assess for arrhythmogenic causes of his SCD (such as CPVT), which was negative.
For patients who develop end-stage heart failure from ischaemic cardiomyopathy, heart transplantation can be considered. Although there are documented cases of coronary artery bypass graft (CABG) surgery in single vessel hypoplasia [9], this is not commonplace. Moreover, CABG may not be feasible when there is diffuse disease [5].