ABSTRACT
ABSTRACT
Background: The term phyllodes tumours, which account for less than 1% of breast neoplasms, describes a spectrum of heterogenous tumours with different clinical behaviours. Less than 30% present as metastatic disease. Complete surgical resection is the standard of care so that recurrence rates are reduced. The role of adjuvant chemotherapy or radiation therapy is controversial. Patients with metastatic disease have a median overall survival of around 30 months.
Case description: The authors present the case of a 57-year-old woman with an exuberant left malignant phyllodes tumour with bilateral involvement, as well as lung and axillar metastasis. The patient underwent haemostatic radiation therapy and started palliative chemotherapy with doxorubicin, achieving partial response with significant improvement in quality of life. A posterior simple mastectomy revealed a small residual tumour.
Discussion: Metastatic malignant phyllodes tumours are rare, so therapeutic strategies rely on small retrospective studies and guidelines for soft tissue sarcoma. Palliative chemotherapy protocols include anthracycline-based regimens, either as monotherapy with doxorubicin or doxorubicin together with ifosfamide. With few treatment options, management of these patients must rely on a continuum of care.
LEARNING POINTS
- Phyllodes tumours are a rare type of breast neoplasm.
- The differential diagnosis of breast cancer should include phyllodes tumours.
- Accurate and rapid diagnosis is required
KEYWORDS
Phylloid, tumor, breast, cancer
CASE DESCRIPTION
A 57-year-old woman, without a relevant medical history, noticed a 2 cm wide lump in her left breast in early 2017 but was not concerned. The breast mass progressively increased until August 2019, when she presented to an emergency department for uncontrolled pain and accepted medical care.
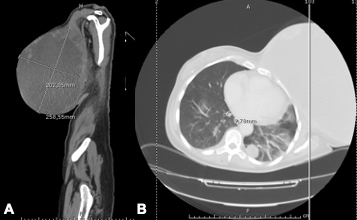
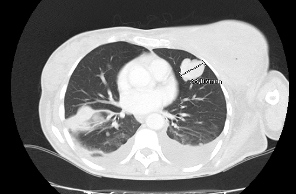
The patient underwent diagnostic investigation, including a left breast CT-guided biopsy in September 2019 with histological results compatible with a mesenchymal tumour. A thoraco-abdominopelvic CT scan revealed full occupation of the left breast area by a large tumoural formation measuring 258 mm at its widest diameter with a mostly liquid component with peripheral areas of tumour heterogeneity (Fig. 1). In addition to two left enlarged axillary lymph nodes about 1 cm in diameter, at pulmonary level there were several bilateral metastatic nodules, the largest being 41 mm in diameter (Fig. 2).
Figure 1. Initial CT scan showing a heterogenous mass: (A) sagittal plane; (B) transverse plane
Figure 2. CT scan revealing lung metastases, the largest 41 mm in diameter
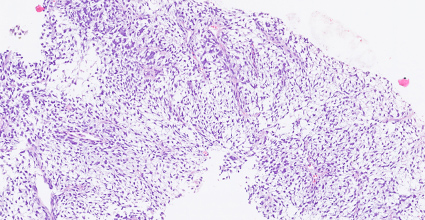
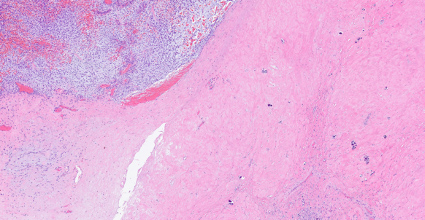
A lung biopsy (Fig. 3) was performed, as well as a confirmatory breast biopsy. Tissue samples were negative for epithelial markers (AE1/AE3, cytokeratin 5/6, cytokeratin 19), P63, oestrogen receptors, S100 protein and smooth muscle actin, but strongly positive for vimentin, so the histological report was compatible with a malignant phyllodes tumour with lung metastasis.
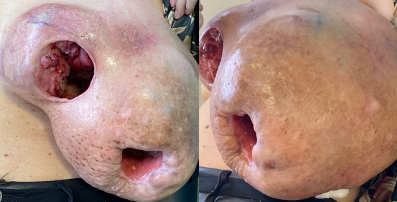
At her first medical oncology appointment in November 2019, the patient had an Eastern Cooperative Oncology Group (ECOG) Performance Status of 0. She presented with a large, hard exophytic mass measuring 35 cm at its widest diameter, with three large ulcerative bleeding lesions (Fig. 4), the biggest having a depth of 8 cm and smelling of putrefaction. Right breast examination revealed several small nodules.
Blood work-up revealed grade 4 anaemia (haemoglobin level 5.7 g/dl). Tumour markers were within the normal range: CEA 0.82 ng/ml (normal range <3.4 ng/ml) and CA 15.3–22.1 U/ml (normal range <35 U/ml).
Figure 3. Haematoxylin and eosin staining of a lung biopsy specimen showing fusiform cells within myxoid stroma, as well as moderate cytological atypia with rare mitoses
Figure 4. Large exophytic lesion occupying the entire left breast area with three ulcerative lesions
The patient was admitted to the oncology ward after blood transfusion support with 2 units of packed red blood cells. On the second day of her hospital stay, she started haemostatic radiotherapy (13 Gy/2 fractions) with a progressive decrease in blood transfusion support needs. As a result, she was able to start first-line palliative chemotherapy with doxorubicin at a dose of 70 mg/m2 on 9 December. She was discharged after 16 days of hospitalization and continued chemotherapy in an outpatient clinic.
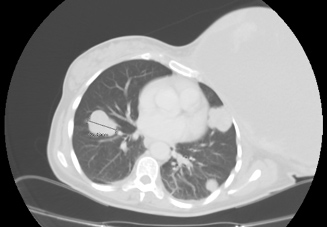
Tumour assessment by CT scan was performed after four cycles and revealed a heterogenous response, with a slight reduction in the primary tumour with increased gaseous component, but with progression of lung metastasis, the largest nodule being 9 cm in diameter, with bilateral pleural effusion (Fig. 5).
In view of the local response, the patient continued with doxorubicin for two more cycles with a cumulative dose of 420 mg/m2. Throughout the course of treatment, she reported grade 1 nausea, vomiting and mucositis, as well as grade 1 anaemia, without further need for blood transfusions.
Tumour assessment after six cycles of chemotherapy revealed a partial response of the primary tumour and lung lesions.
Since April 2020, the patient has been under active surveillance and the breast lesion has been regressing, with one of the three initial ulcerative lesions fully resolved.
As a CT scan in July 2020 showed steady disease with the left breast mass measuring 29 cm, a left mastectomy was proposed to the patient in order to improve her quality of life. A simple left mastectomy was performed in September 2020 with removal of a 1714 g mass measuring 19×17×10 cm. The histopathology report revealed a heterogenous lesion with an area of malignant lesion measuring only 1 cm at its largest diameter and 2 cm from the surgical margin, with an epithelial component surrounded by abundant fibrotic tissue. The final diagnosis was compatible with a phyllodes tumour (Fig. 6).
Figure 5. CT scan after four cycles of chemotherapy showing lung disease progression
Figure 6. Haematoxylin and eosin staining of a tumour fragment from mastectomy tissue with an epithelial component
DISCUSSION
Phyllodes tumours are a heterogeneous group of fibroepithelial tumours with diverse biological behaviours, and range from benign fibroadenomas (around 50%) to malignant invasive phyllodes tumours (less than 30%) that can degenerate histologically into sarcomatous masses [4, 10, 11].
Considering its distinct biological behaviour, histologically, phyllodes tumours can be classified as benign, borderline or malignant based upon four characteristics: the degree of stromal cellular atypia, mitotic activity, infiltrative or circumscribed tumour margins and stromal overgrowth. The last feature is the most strongly related to metastatic behaviour [1, 12, 13].
Complete surgical excision is the standard of care for phyllodes tumours in order to reduce local recurrence rates. Spitaleri et al. conducted a multivariate analysis with 172 patients with phyllodes tumours and concluded that a positive margin (despite lack of its definition) is associated with an almost fourfold higher risk of a tumour-related event, such as local recurrence or distant disease (hazard ratio 3.9, 95% CI 1.2–14.3) [6]. However, new evidence suggests that a positive surgical margin is a concern mostly in malignant phyllodes tumours, as a recent meta-analysis of 54 retrospective studies with 9234 patients found a significantly increased risk of local recurrence only for malignant phyllodes tumours [14]. Consequently, there is ongoing debate about management approaches to a positive surgical margin. Moreover, if adequate margins can be achieved, breast-conserving surgery and mastectomy are both effective options for borderline and malignant tumours regarding disease-free survival [15] and cause-specific survival irrespective of tumour size [16].
Despite surgical outcome, phyllodes tumours recur locally depending on tumour grade, with a meta-analysis reporting local recurrence rates of 8%, 13% and 18% for benign, borderline and malignant tumours, respectively [14].
Axillary lymph node involvement is rare, even when tumours are malignant, so axillary dissection is not mandatory [16].
Adjuvant radiotherapy reduces the local recurrence rates of borderline and malignant phyllodes tumours, but does not affect overall or disease-free survival. The effect on local recurrence after mastectomy is less pronounced (HR 0.68, 95% CI –0.28 to 1.64) compared with breast-conserving surgery [8].
The role of adjuvant chemotherapy is controversial, mainly due to the fact that malignant phyllodes tumours have a better prognosis than most high-grade sarcomas of a similar stage and there are no randomized control trials.
Metastatic disease is reported in 13–40% of patients with phyllodes tumours [1, 4] with a median overall survival of 30 months, which is affected by mitotic activity and stromal overgrowth and differentiation into osteosarcomatous or chondrosarcomatous features [11]. Phyllodes tumours metastasize mostly to the lungs and, similarly to soft tissue sarcomas, pulmonary metastases should be resected when technically feasible. The choice of chemotherapy schemes in this setting is based upon treatment guidelines for soft tissue sarcoma. For patients with a good performance status and minimal comorbidity, doxorubicin with or without ifosfamide is the first-line chemotherapy regimen. Combined therapy is mainly prescribed for symptomatic patients with a need for quick tumour shrinkage. Other single-agent chemotherapy schemes include dacarbazine or ifosfamide [11].
The randomized phase III GeDDis trial compared gemcitabine plus docetaxel versus doxorubicin as first-line treatment in previously untreated advanced unresectable or metastatic soft tissue sarcomas [17]. This trial showed progression-free survival and overall survival similar to doxorubicin. However, gemcitabine-based chemotherapy is an option for patients with contraindication for anthracycline prescription.
Phyllodes tumours show expression of oestrogen and progesterone receptors, but largely in the epithelial component and not the stromal component, so that hormone therapy is not effective against the main driver of metastatic behaviour [18].
The authors have reported a case of a giant metastatic phyllodes tumour treated with first-line chemotherapy with doxorubicin, a regimen mostly studied for soft tissue sarcomas. Because of severe anaemia at first evaluation, as well as a lack of robust evidence for second-line chemotherapy protocols, our team decided to withhold ifosfamide for first progression and proposed the patient for mastectomy in order to improve her quality of life. Mastectomy ultimately revealed a small breast fibrosarcoma only 1 cm in width. As previously discussed, there are limited data regarding these types of tumours, with most evidence obtained from small retrospective studies, so that management of these patients must rely on a continuum of care.