ABSTRACT
The development of Clostridium difficile infection in COVID-19 patients is an understudied complication of the disease. Herein, we present the case of a 46-year-old man who developed severe healthcare-associated C. difficile infection leading to toxic megacolon and perforation in the setting of COVID-19 infection. It is important to continue to follow guidelines regarding antibiotics in healthcare settings to prevent such complications.
LEARNING POINTS
- Co-infection with Clostridium difficile and COVID-19 leads to poor outcomes with high mortality.
- C. difficile infection should be ruled out in COVID-19 patients who develop diarrhoea on antibiotic therapy.
- We should continue to follow the established guidelines of antimicrobial stewardship and remain vigilant for unexpected adverse effects.
KEYWORDS
COVID-19, antibiotics, microbial resistance, Clostridium difficile
INTRODUCTION
As infection control measures have increased, we have seen a reduction in hospital-acquired infections including healthcare-associated Clostridium difficile infection (HA-CDI). Although COVID-19 and C. difficile infection (CDI) are rarely reported together, the association has been linked to severe complications [1]. Diarrhoea is one of the most common non-pulmonary presenting symptoms seen with COVID-19 [2]. This can act as a confounding factor and delay diagnosis and treatment of CDI leading to the worst outcomes.
CASE DESCRIPTION
A 46-year-old-man with a medical history of obstructive sleep apnoea presented to an outside hospital with shortness of breath and diarrhoea. He subsequently had a positive RT-PCR nasopharyngeal swab test and was diagnosed with COVID-19 pneumonia. Remdesivir, convalescent plasma and methylprednisolone were commenced. Given the high leucocytosis (13.7×103/µl) and elevated procalcitonin (3.2 ng/ml), the patient was started on intravenous (IV) vancomycin and piperacillin/tazobactam for empiric coverage for bacterial pneumonia. On day 5, he was intubated for acute hypoxic respiratory failure on the mechanical ventilator and was transferred to our facility for a higher level of care.
On presentation, his blood pressure was 92/55 mmHg, heart rate was 63 beats/minute and temperature was 37.3°C. He had an elevated white cell count (19.8×103/µl), creatinine of 0.7 mg/dl and normal liver function tests. Blood culture from the outside hospital showed no growth and antibiotics were discontinued. A computed tomography (CT) angiogram of chest was negative for pulmonary embolism. Steroids were continued.
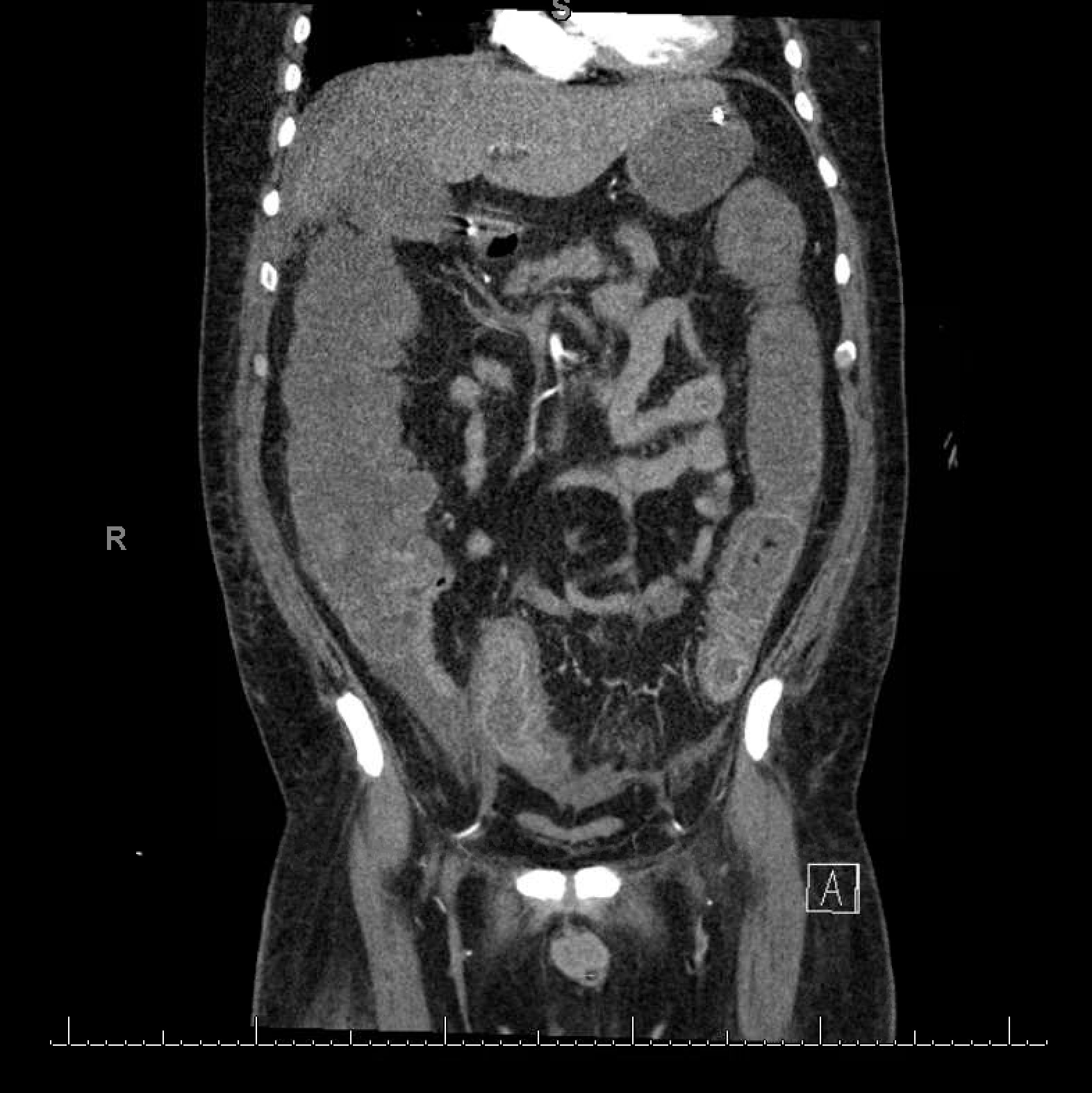
On day 7, the patient’s feeding tube was dislodged leading to an aspiration event. This resulted in septic shock requiring vasopressors and he was restarted on IV piperacillin/tazobactam. In the next 48 hours, acute elevation in the white cell count from 15.4×103/µl to 32×103/µl was noted. Lactic acid increased to 4.2 mmol/l. The patient became oliguric and creatinine rose to 3.21 mg/dl requiring continuous renal replacement therapy. He was noted to have profuse diarrhoea and a C. difficile PCR test was positive. For further work-up, a CT scan of the abdomen and pelvis was done which showed evidence of pancolitis (Fig. 1). Oral vancomycin with IV metronidazole was initiated and piperacillin/tazobactam was discontinued.
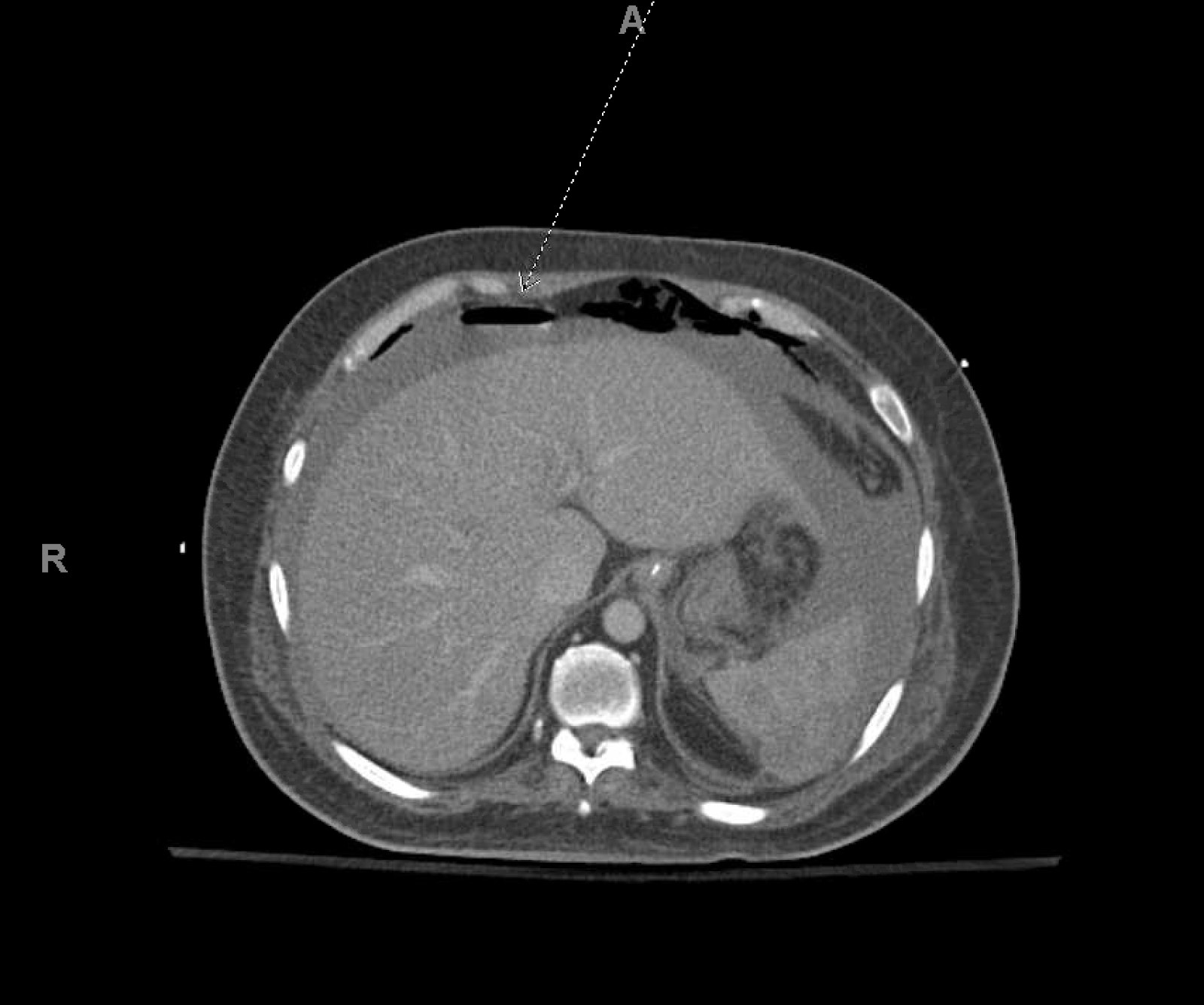
On day 11, the patient’s vasopressor requirement abruptly increased. His leucocytosis increased to 57.4×103/µl, and his lactate was 6.0 mmol/l, suggesting the development of toxic megacolon. He underwent an emergency exploratory laparotomy and total abdominal colectomy, performed at the bedside due to COVID-19 precautions. His abdomen was closed following repeat exploratory laparotomy and end ileostomy 2 days later. Overnight, his clinical status acutely worsened with deteriorating metabolic acidosis. He became hypothermic to 35.1°C, his lactate increased to 12.5 mmol/l, arterial pH decreased to 7.15, and haemoglobin decreased to 6.4 g/dl, requiring the transfusion of 4 units of red blood cells. CT of the abdomen/pelvis revealed haemoperitoneum (Fig. 2). The patient underwent four additional surgeries for the management of persistent haemorrhagic shock, which was further complicated with disseminated intravascular coagulation requiring further resuscitation with blood products.
Figure 1. Computed tomography scan showing diffuse mural thickening and oedema of the colon, especially involving the rectum and sigmoid colon consistent with pancolitis
Figure 2. Computed tomography scan of the abdomen and pelvis showing large volume haemoperitoneum (broken arrow)
On hospital day 17, the patient was noted to have ST segment elevations on the monitor. A formal electrocardiogram and serial troponins confirmed the diagnosis of anterior-inferior wall ST elevation myocardial infarction. After discussion with his family, the patient was ultimately transitioned to comfort care and died a few hours later.
DISCUSSION
Diarrhoea is one of the most common non-pulmonary presenting symptoms of COVID-19, being reported in up to 19% of patients [2]. Patients in the intensive care unit (ICU) have a higher incidence of gastrointestinal dysfunction, especially diarrhoea, due to both infective and non-infective causes [3]. These factors make diarrhoea a common symptom in mild, moderate and severe COVID-19 disease, which can act as a confounding factor leading to a delay in the diagnosis of CDI.
The COVID-19 pandemic has placed global emphasis on infection prevention measures. Techniques including but not limited to hand hygiene, surface disinfection, social distancing and the adoption of personal protective equipment have all seen an unprecedented rise over the last year, which in turn has resulted in a declining rate of HA-CDI [4].
The use of antibiotics due to anchoring bias remains prevalent in the management of COVID-19 patients worldwide. Rawson et al. reported that 72% of hospitalized COVID-19 patients received antimicrobial therapy even though only 8% were reported to have a superimposed bacterial/fungal co-infection [5]. Additionally, COVID-19 has the potential to significantly alter the gut microbiota of patients during hospitalization [6]. This places patients infected with COVID-19 with an altered gut microbiome at high risk of various antibiotic-associated adverse effects including HA-CDI [7].
A large-scale retrospective case–control study of 8402 COVID-19 patients found a significant link between antibiotic use and CDI [8]. It showed that patients who developed CDI over the course of their COVID-19 admission were likely to have longer hospital stays and worse outcomes compared with patients who did not develop CDI. Most patients who developed CDI in the setting of COVID-19 were elderly (average age: 79 years) with multiple comorbidities (only 2.6% of patients who developed CDI had no comorbidities). The mortality rate was also significantly higher at 28% among COVID-19 patients with CDI compared with 5% among the general population with CDI [8]. This highlights that CDI should be ruled out if patients develop diarrhoea on antibiotic therapy.
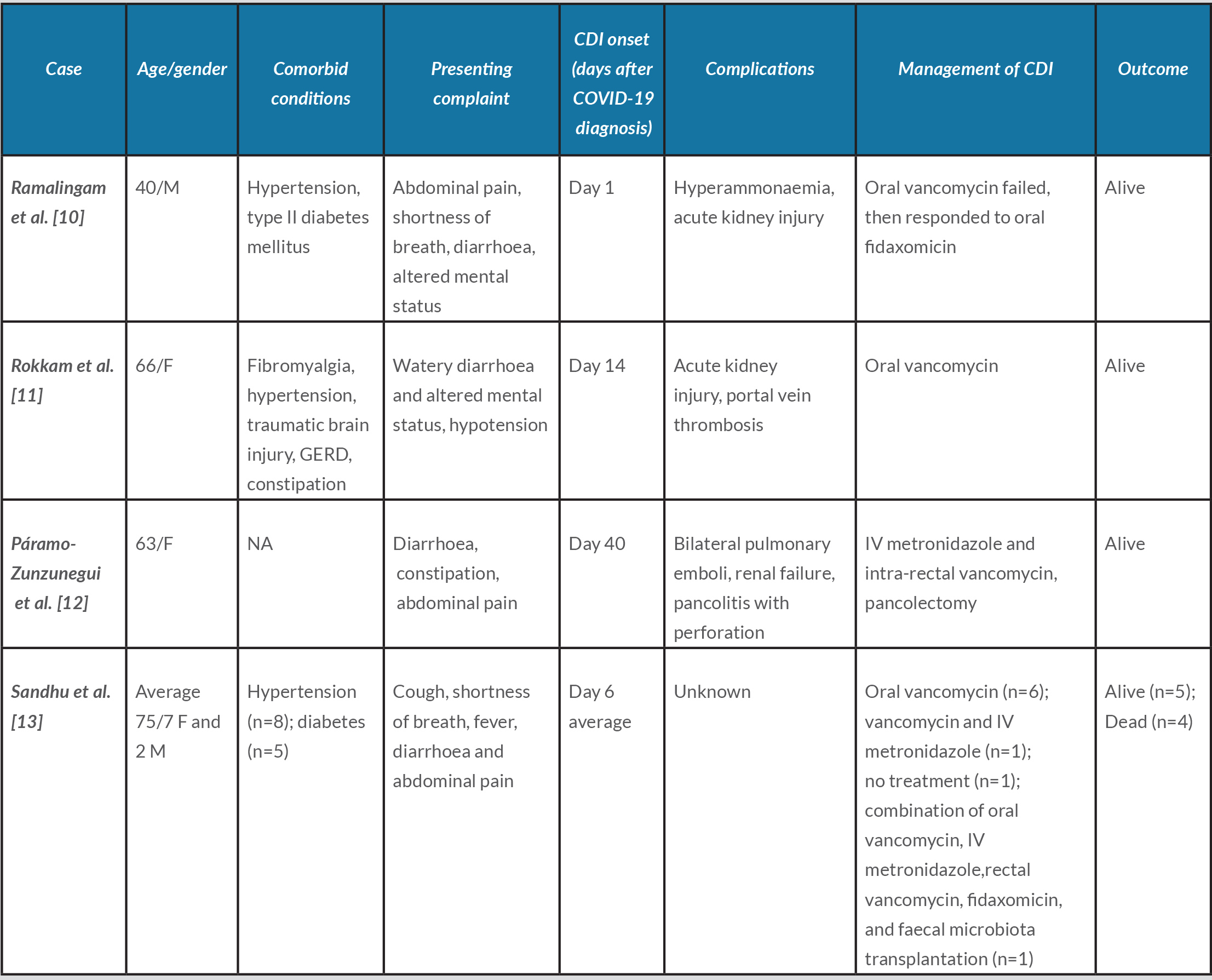
CDI is a challenging disease with a recurrence rate of 15–20% and mortality rate of 5% [9]. While there is a general paucity of data regarding CDI in COVID-19 patients, we found three case reports which make for interesting comparisons with our patient [10–12]. All of these cases, plus the nine subjects discussed by Sandhu et al., emerged in the setting of extensive use of broad-spectrum antibiotics leading to severe CDI, as illustrated in Table 1 [13].
Table 1. All SARS-CoV-2 and Clostridium difficile co-infection cases reported to date
CDI, Clostridium difficile infection; COVID-19, novel coronavirus disease 2019; F, female; IV, intravenous; M, male; n, number/frequency; NA, not applicable.
CONCLUSION
Our case highlights the importance of judicious use of antibiotics in patients with COVID-19 infection. Therefore, we should continue to follow established guidelines of antimicrobial stewardship and remain vigilant for expected antimicrobial adverse effects.