ABSTRACT
Various vaccines against COVID-19 have been developed since SARS-CoV-2 emerged at the end of 2019. Their emergency administration in healthcare settings has been accompanied by numerous adverse effects. A case of Guillain-Barré syndrome following vaccination with Covishield is presented here to highlight this possible adverse condition.
LEARNING POINTS
- Guillain-Barré Syndrome (GBS) is a very rare complication after vaccination against SARS-CoV-2.
- The key concepts related to the understanding, management and outcomes of patients with GBS are discussed.
KEYWORDS
Covishield, Guillain-Barré syndrome, GBS, AstraZeneca, coronavirus, pandemic, vaccine, ChAdOx1
INTRODUCTION
Guillain–Barré syndrome (GBS) is a rare autoimmune disease which can be fatal and whose exact cause is still unknown. A very small number of people can develop GBS following vaccination. The Oxford-AstraZeneca COVID-19 vaccine is distributed under the brand name Covishield in India. It consists of a viral vector of the modified chimpanzee adenovirus ChAdOx1 with COVID-19 spikes present on the outer surface. This protein helps to generate an immune response against SARS-CoV-2. The vaccine has been approved for emergency use by the WHO.
CASE DESCRIPTION
A 52-year-old woman with no comorbidities received her first dose of the Oxford–AstraZeneca Covishield vaccine on 1 April 2021. There was no swelling, redness or pain at the site of injection. The patient developed a fever that evening which continued the following day but eventually resolved. She then complained of a mild headache which lasted for 2 days. On the ninth day after vaccination, she developed lower back pain but was able to walk comfortably. The next day she complained of extreme fatigue and on day 11, she experienced progressive difficulty in walking and moving. She presented to the ER with bilateral lower limb weakness with pain and a burning sensation in the lower back that radiated downwards, was unable to walk without support and complained of limping. She did not have a history of nausea, vomiting, seizures, loss of consciousness, facial weakness or speech alteration. She had no autoimmune conditions, previous cerebrovascular events or cardiac events. She was able to empty her bowels and bladder comfortably. She did not complain of shortness of breath.
On examination, she was haemodynamically stable and maintained oxygen saturation on room air. On neurological examination, she was conscious and well oriented to time, place and person. There were no memory or speech-related deficits, and soft touch and pain sensations were present in all four limbs. Muscle density and tone was normal in both upper and lower limbs with no involuntary movements. Power was reduced to 3/5 in both lower limbs and to 4/5 in both upper limbs, with intact coordination in the upper limbs. Reduced deep tendon reflexes were noted as well as a diminished plantar reflex bilaterally. Cerebellar function seemed to be intact. There was no cranial nerve involvement on examination. All other systemic examinations were within normal limits.
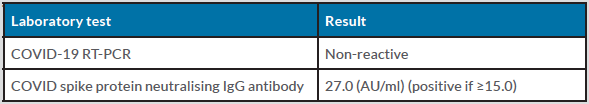
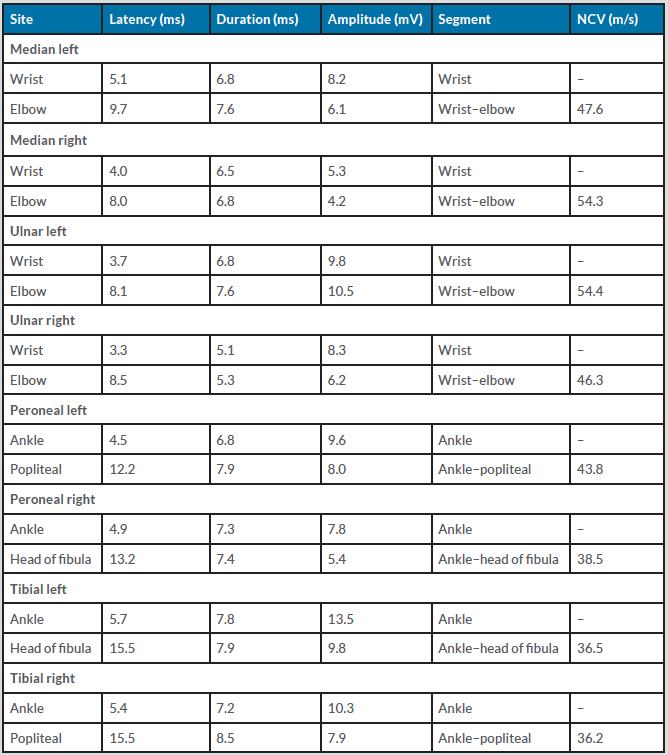
The patient was transferred to the intensive care unit for further management. An MRI scan of the brain and entire spine was inconclusive. A Covid RT-PCR swab was negative (non-reactive) and COVID-19 spike protein neutralising antibody levels were elevated (Table 1), suggesting an immune response due to recent vaccination. We also conducted a nerve conduction study along with electromyography (EMG) which revealed mild demyelinating distal motor neuropathy in all four limbs (Table 2).
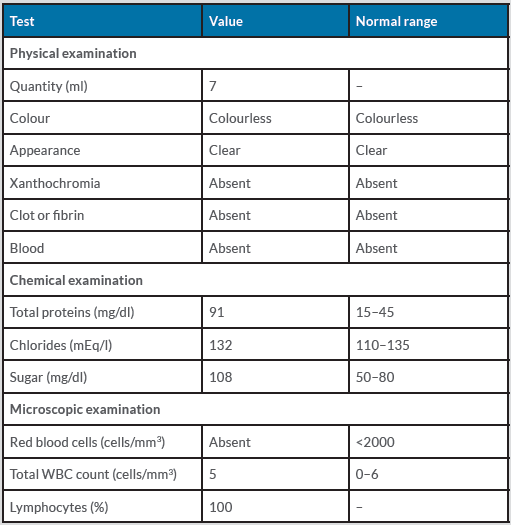
We proceeded with a lumbar puncture under aseptic conditions on 15 April 2021 and sent the cerebrospinal fluid (CSF) for routine investigation and a BioFire PCR meningitis profile. CSF studies reported an elevated total protein level of 91 mg/dl and glucose of 108 mg/dl with a normal leucocyte count of 5 cells/mm3, suggesting the polyneuropathy was likely GBS due to albuminocytological dissociation (Table 3). The BioFire meningitis panel was negative, ruling out meningitis.
Table 1. Initial investigations
Table 2. Nerve conduction study
Table 3. Cerebrospinal fluid (CSF) routine studies
After appropriate consent was obtained, we began management with intravenous immunoglobulins for 5 days, resulting in no improvement. The patient's weakness progressed to 0–1/5 in both lower and upper limbs. The patient was intubated and ventilated because of severe tachypnoea and a tracheostomy procedure was carried out a week later.
As of July, the patient continues to require intensive care treatment. We successfully weaned her from the ventilator and she currently does not require respiratory support and decannulation of the tracheostomy is being planned. However, there has been no significant improvement in power and the patient remains in a quadriplegic state requiring extensive rehabilitation and physiotherapy.
DISCUSSION
Guillain-Barré syndrome is a rare autoimmune condition that is usually appears after infection and results in inflammatory demyelination of nerve fibres. Previous studies conducted during the 1976 swine flu outbreak found GBS developed after vaccination, with 8.8 cases reported per million recipients of influenza vaccine [1].
An association between the Covishield vaccine and GBS has not yet been established. The Covishield vaccine consists of a viral vector of the modified chimpanzee adenovirus ChAdOx1 with COVID-19 spikes present on the outer surface [2]. It is delivered in two doses 4–12 weeks apart [3]. Between April 2020 and December 2020, more than 20,000 individuals participated in clinical trials in the UK, Brazil and South Africa. The results overall concluded that the vaccine was approximately 76% effective in protecting against SARS-CoV-2 and produced an appropriate immune response to prevent hospitalisation and death 22 days after administration of the first dose [2].
A few case reports have been published describing patient who developed GBS within 2 weeks of vaccination. A quick search on PubMed with the keywords ‘GBS’ and ‘vaccine’ revealed two publications. A case report by Nasuelli et al. reported the case of a 59-year-old man who developed four-limb distal paraesthesia and postural instability 10 days after vaccination with ChAdOx1 nCoV-19, which is a component of Covishield. The patient was diagnosed with demyelinating polyradiculoneuropathy on EMG [4].
Another case report published by Maramattom et al. discussed seven patients who developed neurological deficits within 2 weeks of vaccination. All patients were 43–70 years of age, six were female and one was male. Six patients progressed to respiratory failure. It was also reported that one recovered, while others were still hospitalized. All patients progressed to areflexic quadriplegia and four patients developed other cranial neuropathy. It is also worth noting that the authors reported a 1.4–10-fold higher number of cases of GBS than expected in the studied population [5]. Eight case reports comprising 14 patients published in PubMed-indexed journals describe GBS after vaccination [4-11]. Four of these patients developed GBS after receiving an mRNA-based vaccine (Pfizer) [8-11] and 10 after a ChAdOx1-based vaccine (AstraZeneca) [4-7]. Only a few of the millions of vaccinated patients have developed GBS, indicating that this neurological adverse effect is extremely rare.
CONCLUSION
The COVID-19 pandemic has caused major economic problems and huge loss of life. Vaccination is the best way to prevent hospitalisation and death. Oxford and AstraZeneca together have developed Covishield, which showed promising results during clinical trials in reducing the severity of COVID-19 infection [2, 3]. However, the vaccine does have some adverse effects, including the very rare risk of developing GBS. Nevertheless, the benefits of this vaccine clearly outweigh the risks. Further study of GBS developing after vaccination may be required.
This case report aims to highlight the importance of vigilance for GBS and the initiation of prompt investigation and treatment when patients present with such neurological symptoms following vaccination.