ABSTRACT
We report the use of anakinra to treat a case of a 64-year-old man diagnosed with haemophagocytic lymphohistiocytosis (HLH) with neurological involvement. After the administration of intravenous pulse corticosteroid therapy, immunoglobulin and anakinra the patient showed neurological recovery. However, the recovery was complicated by the perforation of a pre-existing bowel diverticulum. The effect of anakinra on bowel inflammation has not yet been clearly established. It can potentially augment bowel inflammation and contribute to the risk of bowel perforation associated with the concomitant use of corticosteroids.
LEARNING POINTS
- Anakinra can potentially augment bowel inflammation.
- The concomitant use of anakinra and corticosteroids may increase the risk of bowel perforation.
- Use of anakinra and corticosteroids in patients with pre-existing gastrointestinal diseases requires vigilant observation for abdominal symptoms.
KEYWORDS
Diverticulitis, haemophagocytic lymphohistiocytosis (HLH), gut, interkeukin-1 (IL-1) receptor antagonist, gastrointestinal diseases
INTRODUCTION
Anakinra is an interkeukin-1 (IL-1) receptor antagonist that has been reported to be effective in the treatment of life-threatening hyperinflammatory disorders such as haemophagocytic lymphohistiocytosis (HLH) [1]. The effects of anakinra on the different types of IL-1 and their effects on the local inflammatory process in the gastrointestinal tract have not been clearly defined. However, the drug belongs to a group of medicines known as biologics (bioengineered drugs that target cytokines or cell surface molecules), which are suspected to increase the risk of bowel perforation [2]. We present a case of an effective treatment response to anakinra for HLH with neurological involvement. However, the recovery was complicated by the perforation of a pre-existing bowel diverticulum.
CASE PRESENTATION
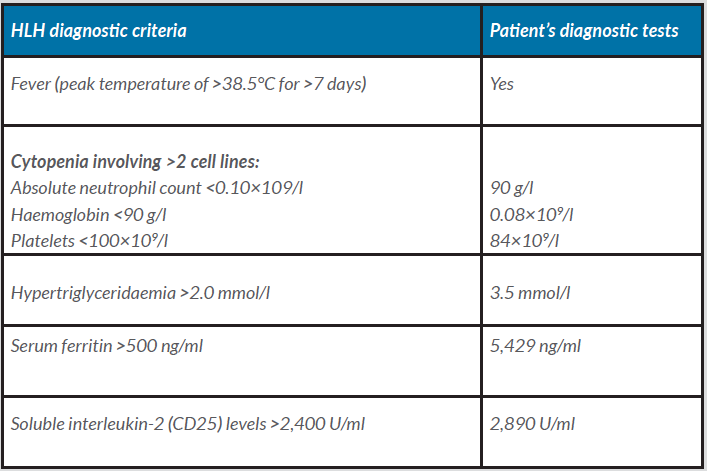
A 64-year-old man was admitted to the internal medicine ward after 2 months of general weakness, significant weight loss, lack of appetite, abdominal pain, constant nausea, dry cough and increased body temperature in the evenings. The immediate cause of admission to the hospital was high temperature (39.0°C), respiratory failure and confusion. Initial empiric antibiotic therapy (cefuroxime) was started soon after admission because of suspected bacterial infection of unknown origin. Chest and abdominal computed tomography (CT) showed diverticulosis and suspicion of mild diverticulitis in the distal colon, slight enlargement of the para-aortic lymph nodes, an enlarged spleen (15 cm) and features of pneumonia. HLH was diagnosed because 6 of the 8 HLH diagnostic criteria were met (Table 1) [3].
Table 1. Haemophagocytic lymphohistiocytosis (HLH) diagnostic criteria and the patient’s diagnostic tests
Treatment began with pulse corticosteroid therapy (1 g of intravenous methylprednisolone) for 3 days and intravenous immunoglobulin (0.4 g/kg/day) for 5 days. During his stay in the internal medicine ward, the patient's condition gradually deteriorated, which manifested as general weakness, poor appetite and worsened consciousness. The treatment was focused on HLH, and the antibiotic therapy was changed to moxifloxacin and ceftriaxone despite the lack of confirmed bacterial infection. The patient could still tolerate feeding and bowel movements were present. Nineteen days after admission to the hospital, chest CT was performed again because of worsening dyspnoea. It excluded pulmonary embolism or pneumonia but revealed free air in the abdominal compartment. Emergency laparotomy was performed during the daytime, but revealed no obvious abdominal pathology. After surgery, the intubated patient was admitted to the intensive care unit (ICU). During the ICU stay, further worsening of neurological symptoms was observed. Cerebrospinal fluid could not be obtained because of severe thrombocytopenia; however, brain imaging revealed no abnormalities. Because of the increased neurological deterioration (deep unconsciousness, muscle flaccidity and no tendon reflexes) and elevated serum ferritin levels (125,000 ng/ml), 100 mg of anakinra was administered subcutaneously, followed by intravenous infusion at 2 mg/kg/day for 7 days. The neurological status of the patient began to improve, and his ferritin levels decreased. In the absence of clinical symptoms of infection and low inflammatory parameter values, the administration of meropenem, initiated in the operating room, was terminated after 10 days of use. The patient was haemodynamically stable and required minimal respiratory support from the ventilator. Enteral feeding, introduced after the surgery, was well tolerated (bowel movements present and no gastric retention), and the total volume of enteral nutrition was gradually increased to 1,200 ml per day. However, 13 days after ICU admission, the patient experienced sudden fever, abdominal pain and an abrupt increase in inflammatory parameters, which was followed by clinical signs of septic shock. Empirical antibiotic therapy with piperacillin (and later vancomycin after confirmation of Enterococcus faecium in blood culture) was initiated. Surgical intervention was performed despite negative findings on abdominal CT. A second laparotomy was performed 14 days after the initial surgery, and revealed faecal peritonitis caused by perforation of a colonic diverticulum. After resection of the affected bowel segment, the patient recovered from septic shock in the ICU.
Examination of resected colon specimens indicated an inflammatory process that was not associated with haemophagocytosis. Finally, the patient returned to the ward after 46 days in the ICU and was discharged home 34 days later.
DISCUSSION
The present case demonstrates the effectiveness of anakinra in the treatment of severe HLH. The presence of free air in the abdominal compartment was probably the result of diverticular microperforation. Microperforation with a distant location of air requires an individualized treatment approach. Some studies suggest that patients with this type of microperforation have a lower chance of successful nonoperative management than those with pericolic air [4]. Furthermore, immunocompromised patients are at a high risk of free perforation and require emergency surgery [5].
In our case, an experienced colorectal surgeon decided on emergency laparotomy despite no clear signs of septic shock or severe peritonitis. However, the macroscopic bowel inspection performed during the surgery could not reveal the location of the perforation. The lack of finding of an impaired bowel suggests that the inflammation process was not strongly exacerbated at this time. Unfortunately, in the later course of illness, diverticulitis developed into full-blown peritonitis, and a second laparotomy was required.
Corticosteroids are known to disturb the intestinal mucosal barrier. This disturbance is probably caused by the inhibition of the cyclooxygenase enzyme in the gut, which is responsible for the synthesis of prostaglandins. The local protective effect of prostaglandins enhances the mucosal barrier of the gut by stimulating mucin and bicarbonate secretion and increasing the local blood flow. The absence of prostaglandins predisposes the mucosa to the effects of noxious agents, such as bacteria and toxins [6]. Initial treatment of HLH with corticosteroids could potentially increase the risk of perforation, as reported in previous studies [7]. A recent meta-analysis demonstrated that the odds of diverticular perforation increased by almost 1 order of magnitude (odds ratio=9) in patients who were administered corticoste
roids [7]. However, a causal relationship was not clearly established.
The use of anakinra has also been shown to modulate the course of the inflammatory processes involved in diverticulitis [8]. Anakinra acts primarily on the IL-1 receptor, inhibiting the effects of IL-1 on the healing and repair of colonic tissue, while preserving the inflammatory effects of the IL-1 receptor [9]. This diverse effect on the different types of IL-1 receptors could explain the severe aggravation of inflammatory bowel disease in patients administered anakinra, described in recently published case reports [10, 11]. Thus, through this mechanism, anakinra could have exacerbated the bowel inflammation that led to colon perforation.
The use of the other representative of biologics, tocilizumab (directed antagonist of the IL-6 receptor), has also been found to correlate with gastrointestinal perforation. However, in the case of tocilizumab the mechanism was linked to the effect of IL-6 inhibition on vascular endothelial growth factor (VEGF) [12].
CONCLUSION
In summary, we present a case of a temporal association between the use of corticosteroids and anakinra and bowel perforation. Although the causal relationships were not conclusive, both medicines are known to affect the defence mechanisms of the gastrointestinal mucosa and could potentially contribute to the inflammatory process. Our patient also presented a history of diverticulitis which is known to be a risk factor for lower gastrointestinal perforation, especially in patients treated with other biologics [2]. We conclude that the concomitant use of anakinra and corticosteroids in patients with pre-existing gastrointestinal diseases requires vigilant observation for abdominal symptoms.