ABSTRACT
Since the beginning of the COVID-19 pandemic, efforts have been made to design safe and effective vaccines against SARS-CoV-2.
Numerous vaccines have been designed and tested in limited clinical trials in various countries. Among them, the Sputnik V vaccine has shown a relatively safe profile and, to our knowledge, has no associated major side effects. We describe the case of a 40-year-old female healthcare worker who developed severe persistent eczematous lesions on the second day after she received the first dose of the Sputnik vaccine. The eczematous lesions were refractory to an antihistamine and persisted at the 1 month follow-up. Severe persistent eczematous lesions should be viewed as a potential side effect of vaccination with the Sputnik V vaccine. Moreover, a severe allergic reaction to a COVID-2019 vaccine may indicate the vaccine is ineffective in the recipient.
LEARNING POINTS
- Vaccination against COVID-19 may be accompanied by rare complications.
- Eczematous lesions can be a side effect of the Sputnik V vaccine.
- A severe allergic reaction to a COVID-19 vaccine may result in decreased vaccine effectiveness in the recipient.
KEYWORDS
COVID-19, Sputnik V, eczema
INTRODUCTION
Since the announcement of the COVID-19 pandemic by the World Health Organization [1] in March 2020, more than 289 million confirmed cases of COVID-19 and approximately 3 million deaths have been recorded [2]. Efforts have been made to design safe and effective vaccines against severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) [3]. Despite having numerous benefits, vaccines in general are not completely risk free. The most common side-effects are usually minor, but some can cause serious and life-threatening adverse effects that are not clearly identified until the vaccine is in common use [4]. This also applies to the new COVID-19 vaccines which were developed rapidly due to concerns about the high transmissibility and overall mortality rate of SARS-CoV-2. As phase I, II and III trials were combined in order to save time, it is important to identify previously unreported adverse effects in vaccine recipients.
Iran began COVID-19 vaccination on 9 February 2021 using Sputnik V, the world’s first registered COVID-19 vaccine. The Moderna, Pfizer-BioNTech (mRNA vaccines) [5, 6] and Sputnik V (rAd26 and rAd5 vector-based vaccine) [7] vaccines all have over 90% efficacy. Sputnik V uses a recombinant adenovirus method (adenovirus 26 and adenovirus 5) to express the SARS-COV-2 spike protein and is currently registered in more than 55 countries [8]. The efficacy of the Sputnik V vaccine is 91.4% by 21 days after the first injection [9] and except in one study where four cases of self-reported anaphylaxis were observed [10], no other serious adverse reactions have been reported so far [8]. In this case report, we describe a case of severe persistent eczema in a recipient of the Sputnik V vaccine, which developed soon after the first injection.
CASE DESCRIPTION
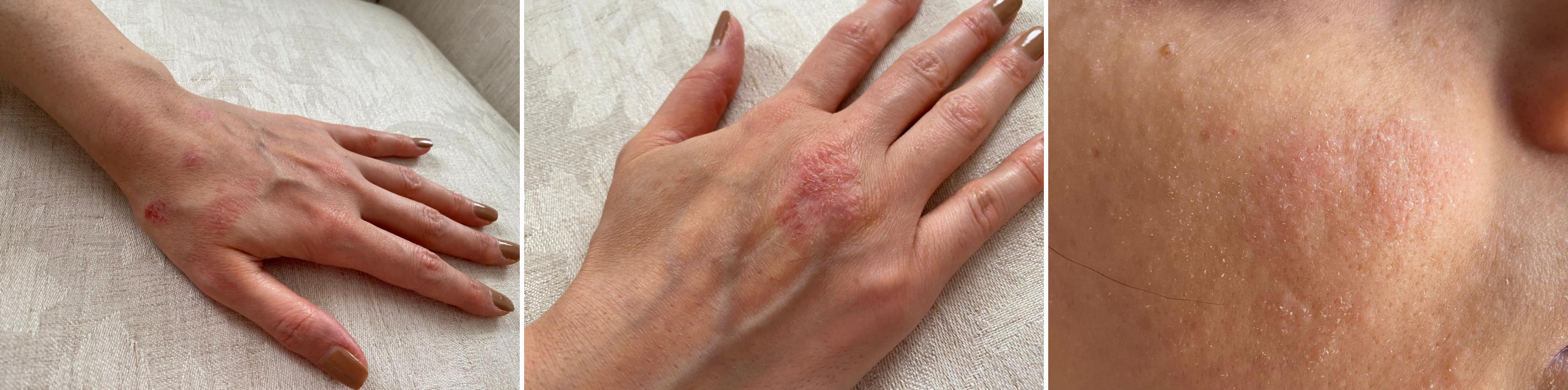
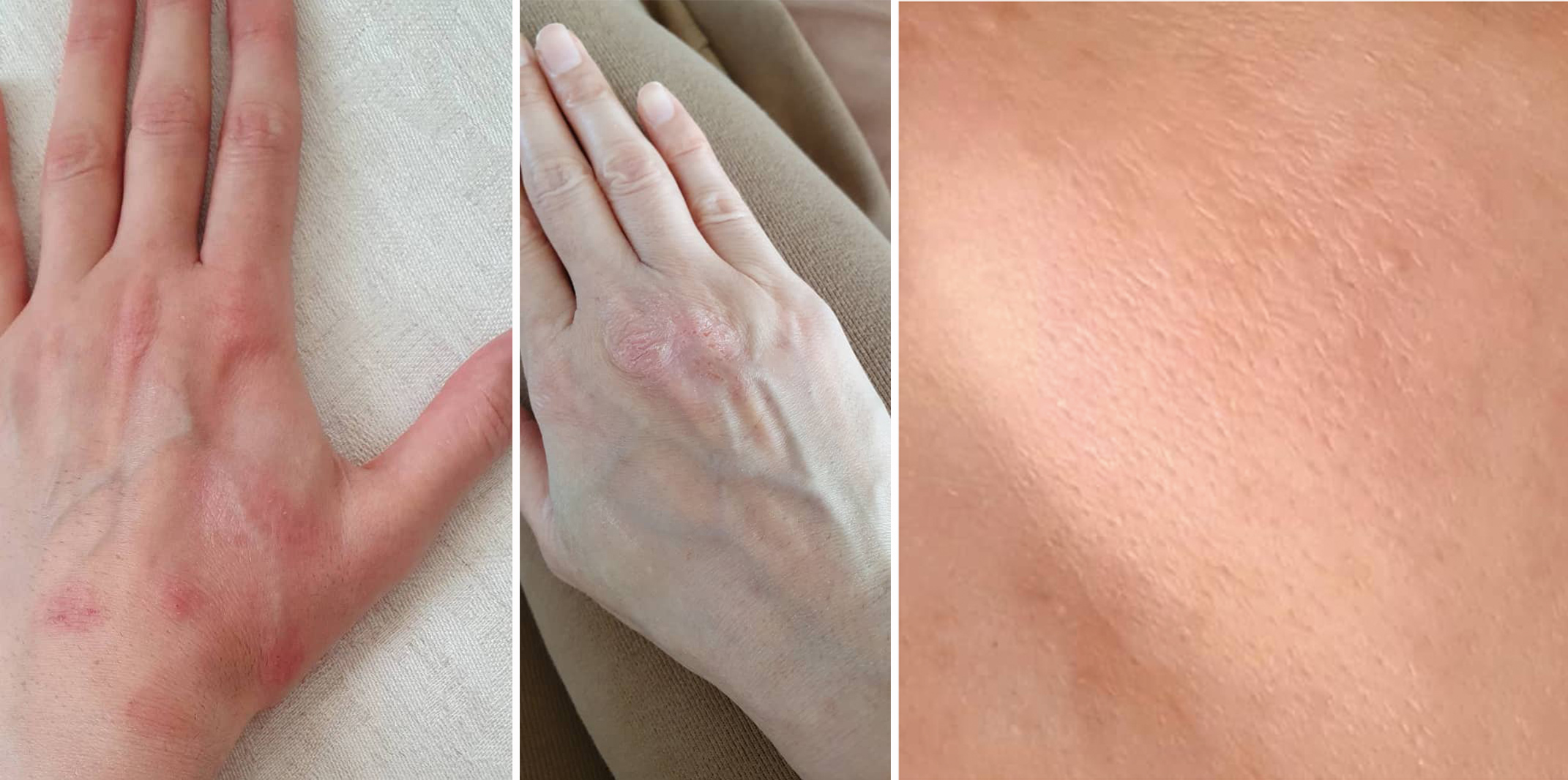
A 40-year-old woman, with no known illness, presented with a 2-week history of eczematous lesions on the forehead, nasal bridge, cheeks, breasts and upper limbs. The patient had received her first dose of Gam-COVID-Vac (Sputnik V) on 24 February 2021. She immediately experienced low-grade fever and mild myalgia. Fever and myalgia subsided on the second day but eczematous-like skin lesions then developed (Fig. 1). She took fexofenadine (Telfast) once daily for 2 weeks as a remedy for the skin rash. Her medical history was insignificant and she was not on any medication. She had no history of COVID-19 infection. She reported no previous allergic reactions to any medication or food. There was no family history of any allergic disease. She was non-smoker, did not drink alcohol, works as a forensics doctor in the hospital and has been exposed to COVID-19 patients. Despite consumption of a second-generation antihistamine (fexofenadine) for 2 weeks, the skin rash was sustained on most of her body except for the face (Fig. 2).
Figure 1. Severe persistent eczematous lesions on the left and right hands and on the cheek 2 days after vaccination
Figure 2. Partially resolved eczematous lesions on the left and right hands, and on the cheek at 1 month follow-up
Early laboratory results did not reveal any pathological findings. There was no leucocytosis or increases in the erythrocyte sedimentation rate (ESR), no uraemia and hepatic enzymes were not elevated. A follow-up laboratory study 1 month later revealed an elevated serum IgE level of 134 (normal range 0–87). Serological study also showed a SARS-CoV-2 IgG antibody index value of 0.2 (positive >1.1) and a SARS-CoV-2 IgM antibody index value of 0.3 (positive >1.1), which indicated no immunity against COVID-19.
DISCUSSION
Negative immunity following the first dose of the Sputnik vaccine has been reported in several recent studies, with the main dermatological side effects being urticarial rashes [10–12]. We are the first to report a severe persistent eczematous reaction following injection of the first dose of the Sputnik vaccine.
Sputnik V is a COVID-19 adenoviral vector vaccine developed by the Gamaleya Research Institute of Epidemiology and Microbiology in Russia. It is a two-vector viral vaccine composed of two adenoviruses [13]. These vector viruses act as a container to carry the gene that expresses the spike protein (a surface protein which is only found in SARS-CoV-2) into human cells [14]. Once the vector virus is inside, it induces the host cell to express the spike protein and displays it on the cell surface, thus stimulating an immune response [14]. The ChAdOx1 nCoV-19 (Oxford–AstraZeneca) vaccine employs the same mechanism of action. Given that our patient had not had any allergic issues previously, it is very likely that the vaccine was responsible for the eczematous lesions. Further studies are needed to establish the safety and efficacy of the Sputnik V vaccine.
CONCLUSIONS
Rare complications of vaccines should be kept in mind during the COVID-19 pandemic. Severe persistent eczematous lesions can occur as a result of vaccination with the Sputnik V vaccine. Moreover, a severe allergic reaction to a COVID-19 vaccine may result in reduced vaccine effectiveness in the recipient.