ABSTRACT
The growing prevalence of obesity in the USA has resulted in increased consumption of weight loss products that promote fat metabolism. Dietary supplements used for weight loss contain a wide variety of ingredients but the amount of scientific information available on these ingredients varies considerably. Such supplements have documented and undocumented adverse effects. Although the FDA frequently issues health advisories, the health consequences of consuming supplements are often overlooked by the general public. A common supplement used for weight loss is Hydroxycut. The ingredients used in the different forms of Hydroxycut products vary but generally include caffeine and green tea extract, which are responsible for a wide range of adverse effects. We present the case of 41-year-old man with a medical history of polysubstance abuse who developed acute compartment syndrome in the setting of rhabdomyolysis from prolonged immobilisation and the use of Hydroxycut. This case demonstrates the possible adverse effects of consuming weight loss herbal supplements like Hydroxycut. Healthcare professionals and consumers are encouraged to report serious adverse events or product quality problems with the use of these supplements to the FDA's MedWatch adverse event reporting program.
LEARNING POINTS
- The weight loss supplement Hydroxycut is sold in various formulations whose side effect profiles are not fully known; the latest formulation has a very high concentration of caffeine which increases the risk of rhabdomyolysis.
- Although uncommon, exercise-induced rhabdomyolysis can cause compartment syndrome, especially in the setting of concurrent use of weight loss supplements.
- High clinical suspicion, prompt diagnosis and early treatment are key to preventing complications from compartment syndrome.
KEYWORDS
Acute compartment syndrome, Hydroxycut, exercise-induced rhabdomyolysis, caffeine
INTRODUCTION
TRhabdomyolysis is caused by muscle cell injury and the breakdown of striated muscle, resulting in swelling and the release of cellular components such as electrolytes, creatinine kinase (CK) and myoglobin, into the blood and urine. Exercise-induced rhabdomyolysis (EIR) is common and rarely requires medical attention, but can sometimes have severe complications including acute kidney injury, hepatic dysfunction, dysrhythmia, heart failure, electrolyte imbalances, compartment syndrome and even death[1]. Although strenuous exercise can cause rapid rhabdomyolysis, it rarely causes acute compartment syndrome[2].
Herbal medicines are widely used in the USA and often unregulated by the FDA. Hydroxycut supplements have been sold for many years with different active ingredients under the same brand name. The product currently contains high levels of caffeine, which may increase the likelihood of rhabdomyolysis.
CASE DESCRIPTION
A 41-year-old hispanic man with a medical history of polysubstance abuse presented to the emergency department (ED) for the evaluation of an unwitnessed syncopal episode. He was found unconscious on the bathroom floor by his stepfather at around 06:00 h; it was unclear how long he had been unconscious. Emergency medical services who arrived at the scene noted he had pin-point pupils and administered naloxone, following which the patient regained consciousness and was brought into the ED for further evaluation. The patient stated that 1 week before presentation he had been investigated for new-onset seizures (multiple episodes) requiring admission to the intensive care unit and intubation. Work-up was inconclusive and the seizures were thought to be secondary to alcohol/drug withdrawal.
Upon arrival at the ED, the patient had a temperature of 36.6°C, heart rate of 103/min, blood pressure of 131/83 mmHg, and respiratory rate of 21/min, saturating well on room air.
He reported pain in the left lower back, buttock and thigh, along with left lower extremity paraesthesia since his fall in the bathroom. He also noted severe pain when attempting to move the left lower limb. In addition, he admitted to daily use of the weight loss supplement Hydroxycut and had engaged in strenuous resistance training at the gym in the days preceding presentation. The review of systems was otherwise remarkable. Physical examination revealed a left lower extremity motor strength of 3/5, and decreased sensations over the entire left extremity. Deep tendon reflexes were graded 1+. Tenderness was present over the left lower back and the left gluteal region.
The initial laboratory results were remarkable for leucocytosis of 18,000/mm3, creatinine of 2.91 mg/dl, anion gap of 23, and creatinine phosphokinase (CPK) levels elevated at 857 U/l.
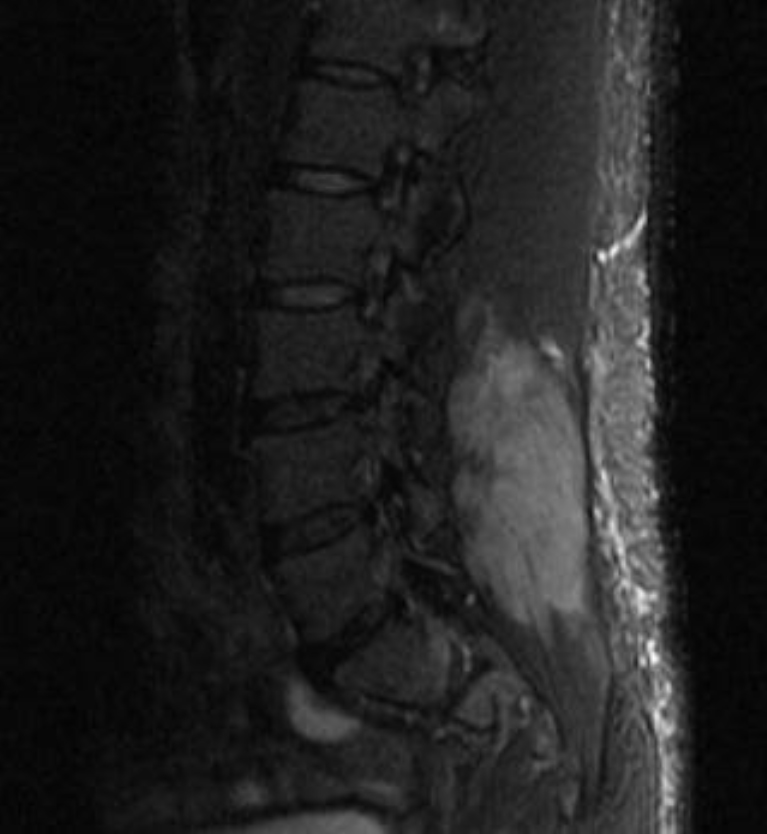
CT of the head performed in the ED was within normal limits. The patient was loaded with levetiracetam and started on IV fluids and admitted for further work-up. Due to the complaints of low back pain and the neurological findings described above, MRI of the spine was ordered, which revealed extensive oedema within the bilateral posterior paravertebral, left obturator externus, and left gluteus musculature as seen in Fig. 1.
Figure 1. MRI of the spine showing extensive oedema within the bilateral posterior paravertebral, left obturator externus, and left gluteus musculature
EThe following day, the patient complained of worsening left lower extremity pain and paraesthesia. Physical examination revealed increased swelling of the left lower extremity, taut and shiny overlying skin, weakness, very painful active and passive ranges of motion, and intact peripheral pulses. CPK levels were noted to be extremely elevated at 172,519 U/l. The patient was immediately transferred to the ICU due to concern about evolving compartment syndrome. Compartment pressures were confirmed by the surgical team to range between 40 and 50 mmHg in the anterior thigh and lower leg compartments. The patient underwent emergency mediolateral calf and anterior thigh fasciotomy. His postoperative course was uncomplicated. CPK reduced to normal levels with IV fluids, and creatinine stabilized at 1.9 mg/ml. Surgery recommended wound closure in 2–3 weeks. The patient was discharged to subacute rehabilitation with appropriate follow-up provided.
DISCUSSION
IAbout one in five adults in the USA admit using a herbal supplement, with about 58% failing to mention their supplement use to their primary care physician[3].
Hydroxycut is a very common supplement because it has been deemed safe for weight loss. Although the market for dietary supplement in the USA is worth billions of dollars, they are widely unregulated by the FDA unless serious complications are reported. In both 2004 and 2009, the FDA withdrew Hydroxycut products containing ephedra from the US market due to multiple health problems including rhabdomyolysis, cardiovascular risks, hepatotoxicity, and even one death. Nevertheless, Hydroxycut products with different active ingredients, most specifically caffeine, are still commercially available[4] as serious complications are rare.
Caffeine overdose has been implicated in rhabdomyolysis, which may have contributed to the adverse effects of Hydroxycut in this case. A possible mechanism of action is that caffeine increases intracellular calcium concentrations by activating the inositol trisphosphate IP3 pathway. This in turn can cause damage to the sarcoplasm and then cells, and rhabdomyolysis can occur. Therefore, caffeine consumption can be a risk factor for rhabdomyolysis[5]. Hydroxycut product labels declare they contain 500 mg or less caffeine per serving, but the recommended dosages can have as much as 900 mg of caffeine[6].
This patient’s clinical picture was multifactorial. The use of Hydroxycut in the presence of a strenuous exercise regime most likely caused this patient’s condition. However, the patient’s history of being unconscious on his bathroom floor for many hours, as well as prior alcohol consumption could have also contributed to rapid rhabdomyolysis and thus acute compartment syndrome.
In summary, the use of the caffeine-containing weight loss supplement, Hydroxycut, poses a signification health risk to the general population. Although the FDA is aware of adverse effects associated with past Hydroxycut formulations, Hydroxycut remains on the market with different active ingredients. Whether the presentation of acute compartment syndrome was related to prolonged immobilization after the syncopal event, or the regular use of Hydroxycut and strenuous exercise, cannot be determined.
CONCLUSION
Dietary supplements are readily available on the market and widely used by the general public. However, they can have dangerous side effects given the high doses of some substances they contain, such as caffeine. It is therefore imperative that medical providers and the general public are aware of the risks associated with dietary supplements. We urge our readers to report cases of related adverse events.