ABSTRACT
We present the case of a 59-year-old man with acute B19 parvovirus infection who developed a systemic inflammatory reaction similar to adult-onset Still's disease (AOSD). We discuss the clinical challenge due to overlapping symptoms to distinguish between a primary B19 viral infection and the autoimmune disease it can trigger.
LEARNING POINTS
- Distinguishing between primary B19 parvovirus infection and autoimmune diseases can be difficult in view of the significant symptom overlap.
- In our patient, recurrence of symptoms during follow-up and response to treatment were in favour of adult-onset Still's disease triggered by B19 parvovirus.
KEYWORDS
Parvovirus B19, adult-onset Still's disease, systemic inflammatory response
INTRODUCTION
Parvovirus B19 is a DNA virus that only infects humans. Infection can range from an asymptomatic condition to life-threatening disease [1]. More than half of patients have non-specific flu-like symptoms such as myalgias or fever, or are asymptomatic. Children present mainly with erythema infectiosum, while osteoarticular symptoms are the most common manifestations in adults, showing clinical features similar to those found in autoimmune connective tissue diseases [2–6]. Its pathogenesis is determined by its special tropism for erythroid progenitor cells, causing their destruction and thus interfering with erythropoiesis, leading to a reduction in haematocrit as one of its most frequent clinical manifestations [1]. Endothelial cells have been recognized as targets for B19 virus infection, and although the causal relationship between B19 virus and myocarditis remains controversial, several studies have concluded that there is a relationship as DNA virus has been found in the heart at autopsy [7].
The origin of the joint and dermatological symptoms is not well established. Both types of symptoms generally coincide with measurable serum antibody production and are therefore presumed to be at least partially immune mediated. However, a direct cytotoxic action of the virus may also play a role [8, 9].
Adult-onset Still's disease (AOSD) is a rare multisystem inflammatory disorder with a wide range of clinical manifestations, predominantly fever, arthritis and evanescent rash [10]. Its aetiology is multifactorial, involving genetic and infectious factors. It has been hypothesized that it could be a reactive syndrome, where certain viral or bacterial infections could act as triggers of the disease in a genetically predisposed host, although so far this relationship has not been conclusively established [11–13].
In addition, some of the clinical manifestations of AOSD are similar to those seen in certain viral infections, making it difficult to make a differential diagnosis between a primary viral infection and a possible autoimmune reactive syndrome as a consequence of AOSD[10–14]. Indeed, several microorganisms, especially viruses, have been associated with juvenile and AOSD.
CASE DESCRIPTIONS
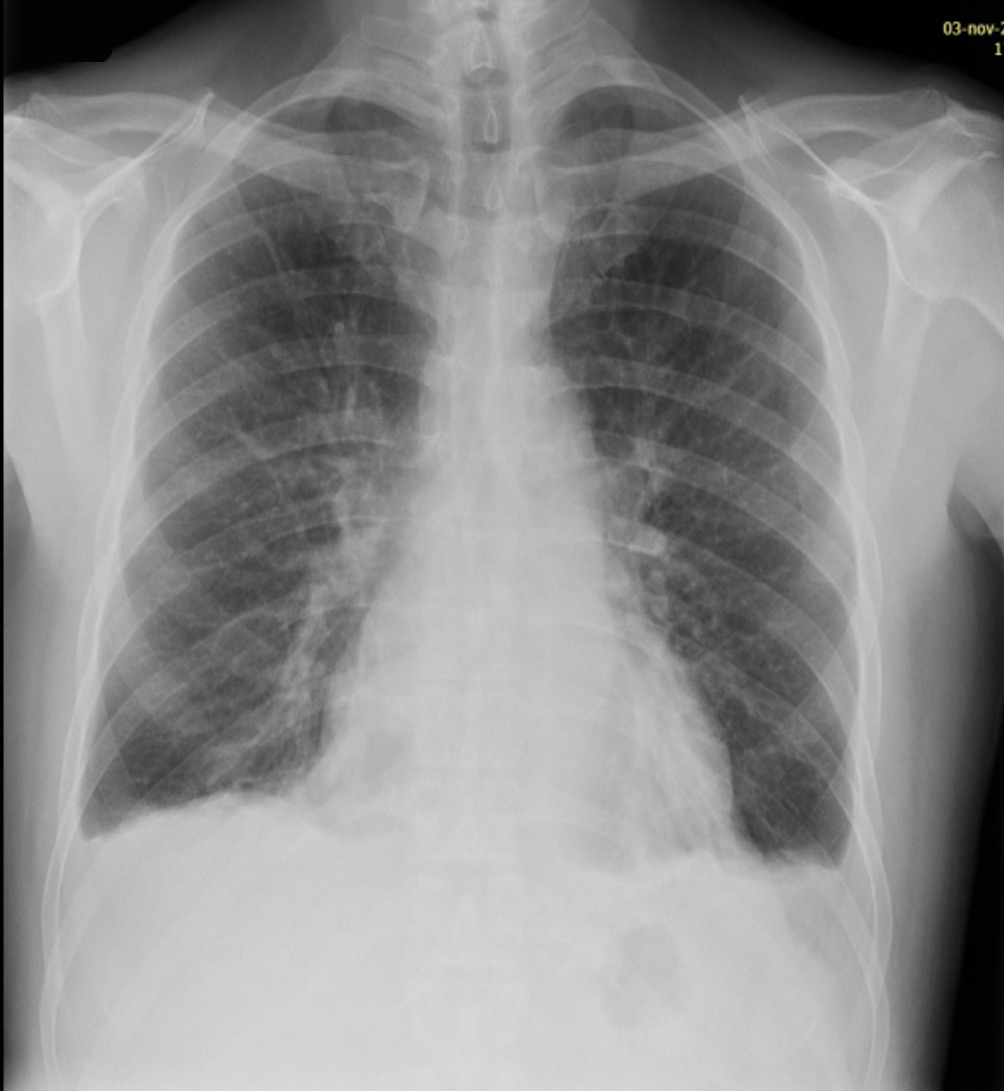
A 59-year-old man with no relevant medical history was admitted to the Internal Medicine Department for a 9-day history of general malaise associated with fever and arthralgias mainly in small joints (carpals and ankles). This clinical picture was preceded by a sudden maculo-papular, non-pruritic rash, predominantly in the upper trunk area, which disappeared within 24 hours, coinciding with the onset of fever up to 39°C. The physical examination revealed the presence of bilateral malleolar oedema. Table 1 shows the laboratory results obtained upon admission. The patient was diagnosed with acute B19 parvovirus infection based on the presence of both IgM and the development of IgG antibodies for this virus. The chest X-ray showed bilateral pleural effusion (Fig. 1).
Table 1. Laboratory investigations
Figure 1. Chest x-ray showing bilateral pleural effusion
A transthoracic echocardiogram was ordered. This showed generalized hypokinesia of the left ventricle with decreased left ventricular ejection fraction (45%) and moderate pericardial effusion. Myocardial markers were normal. During follow-up, the patient presented an acute onset atrial fibrillation but remained haemodynamically stable throughout.
The patient was treated with diuretics, ACE inhibitors, high doses of aspirin and colchicine. Oral anticoagulation was started due to the persistence of flutter. The patient had an initial favourable evolution with improvement of general symptoms and the polyarticular signs, negative IgM and positive IgG for B19 virus, and recovery of left ventricular function in the echocardiogram performed afterwards. However, the articular manifestations and fever reappeared, which led us to consider the presence of a systemic disorder. The patient fulfilled all major and two of the minor Yamaguchi criteria for Still’s disease. In this clinical setting, corticosteroids at doses of 20 mg of prednisone daily in a decreasing regimen were initiated. The patient improved clinically and corticoids were stopped.
DISCUSSION
We present the case of a 59-year-old man with cutaneous exanthema, oligoarthritis, fever, hepatitis and myopericarditis (as pericardial effusion with ventricular dysfunction was present even though troponin levels were normal). Laboratory tests showed elevated acute phase reactants, with positive IgM and subsequently IgG serology for parvovirus B19. The main initial diagnosis was systemic inflammatory reaction in the context of primary infection by parvovirus B19. This diagnosis was initially supported by the early resolution of clinical manifestations. However, the recurrence of symptoms and the need for oral corticoids led us to consider the presence of a systemic inflammatory reaction like AOSD.
There are numerous studies stating that viral infections, such as SARS-CoV-2, can lead to a multisystem inflammatory syndrome. Although the aetiology of this syndrome is not yet clearly known, it is thought to be due to activation of autoantibodies rather than to the cytopathic effect of the virus itself [15]. It has been proposed, in fact, that viruses may act as triggers for many of the known autoimmune diseases. It is therefore important to carry out an exhaustive analysis in order to make an adequate differential diagnosis between both possibilities, although in many cases it is not possible to reach a definitive diagnosis.
The case presented met epidemiological, clinical and serological criteria for the diagnosis of primary B19 virus infection: fever, exanthema, arthritis and positive IgM serology for B19 virus. The role of B19 virus as a causative agent of myocarditis continues to be discussed in the literature [14–17].
The presence of leucocytosis with neutrophilia, hyperferritinemia above 500 and reactive thrombocytosis pointed more towards a possible alternative diagnosis, such as AOSD. The patient fulfilled all major and two of the minor Yamaguchi criteria for Still’s disease. It is important to note that the diagnosis of AOSD is largely one of exclusion, based on a series of clinical and analytical features in the absence of another cause that may justify them [18, 19]. The recurrence of symptoms and the need for corticoid treatment with subsequent resolution of clinical manifestations support the diagnosis of AOSD triggered by B19 parvovirus.
CONCLUSIONS
B19 virus infection may present a clinical picture similar to that of Still's disease due to activation of the immune system, making the initial differential diagnosis between the two difficult. The clinical course of our patient was in favour of Still's disease triggered by B19 viral infection.