ABSTRACT
Hyperglycaemic hyperosmolar state (HHS) and diabetic ketoacidosis (DKA) features can occur simultaneously in 27% of diabetic emergencies and have a two-fold increased risk of death. Despite the high prevalence of this combination, recommended treatments from leading guidelines may not be compatible with the clinical picture.
A 36-year-old man presented with explicit concurrent HHS and DKA. The recommended treatment with simultaneous insulin and volume repletion was followed but resulted in an excessively rapid decline in serum osmolarity. Hyperosmolar therapy (NaCl 3%) was initiated to mitigate the risk of potentially fatal cerebral osmotic shifts.
The concomitant presence of DKA and HHS leads to a treatment dilemma with a high risk of excessive osmolarity shifts. More evidence is needed, but it is reasonable to initiate tailored treatment to avoid osmolarity reduction rates exceeding the hypernatraemia-based limit of 24 mOsm/l/day. Hyperosmolar therapy can be considered but requires frequent monitoring of electrolytes and osmolarity.
LEARNING POINTS
- Simultaneous hyperglycaemic hyperosmolar state (HHS) and diabetic ketoacidosis (DKA) features occur in 27% of diabetic emergencies and have an almost three-fold increased risk of death.
- Combined HHS and DKA requires simultaneous insulin and volume repletion, which may result in an excessive decline in serum osmolarity. More evidence is needed, but it is reasonable to avoid osmolarity reduction rates above the hypernatraemia-based limit of 24 mOsm/l/day.
- Consider hyperosmolar therapy (NaCl 3%) to mitigate the risk of potentially fatal cerebral osmotic shifts.
KEYWORDS
Hyperosmolar hyperglycaemic state, diabetic ketoacidosis, osmolarity, cerebral oedema, case report
INTRODUCTION
Isolated hyperglycaemic hyperosmolar state (HHS) and diabetic ketoacidosis (DKA) are managed equally in most guidelines. However, this might not be appropriate as pathogenesis and patient characteristics are different. In addition, morbidity and mortality increase substantially when both are present. This case report discusses management and pitfalls in a patient with simultaneous HHS and DKA.
CASE DESCRIPTION
IA 36-year-old man with a medical history of diabetes mellitus type 1 without end-organ damage was brought to the emergency department with intractable vomiting, abdominal pain and progressive lethargy for 3 days. He used subcutaneous injections of 18 U short-acting insulin aspart twice daily. Prior to the development of symptoms, he had intermittent complaints of polyuria and polydipsia without weight loss. He denied drug abuse or excessive alcohol intake.
On examination, the patient was well-groomed, dehydrated, lethargic and disoriented, but responsive to pain. He had an acetone foetor and Kussmaul respiration at 34 breaths per minute, and an axillary temperature of 36.5°C. Vital signs showed a blood pressure of 70/40 mmHg and heart rate of 135 beats per minute, pulse oximetry was 99% on ambient air. There was mild pain upon palpation of the epigastric region, without rebound tenderness. Neurological examination showed reduced consciousness, without nuchal rigidity or pathological reflexes. There were no signs of gingivitis or balanitis. The remainder of the examination revealed no abnormalities.
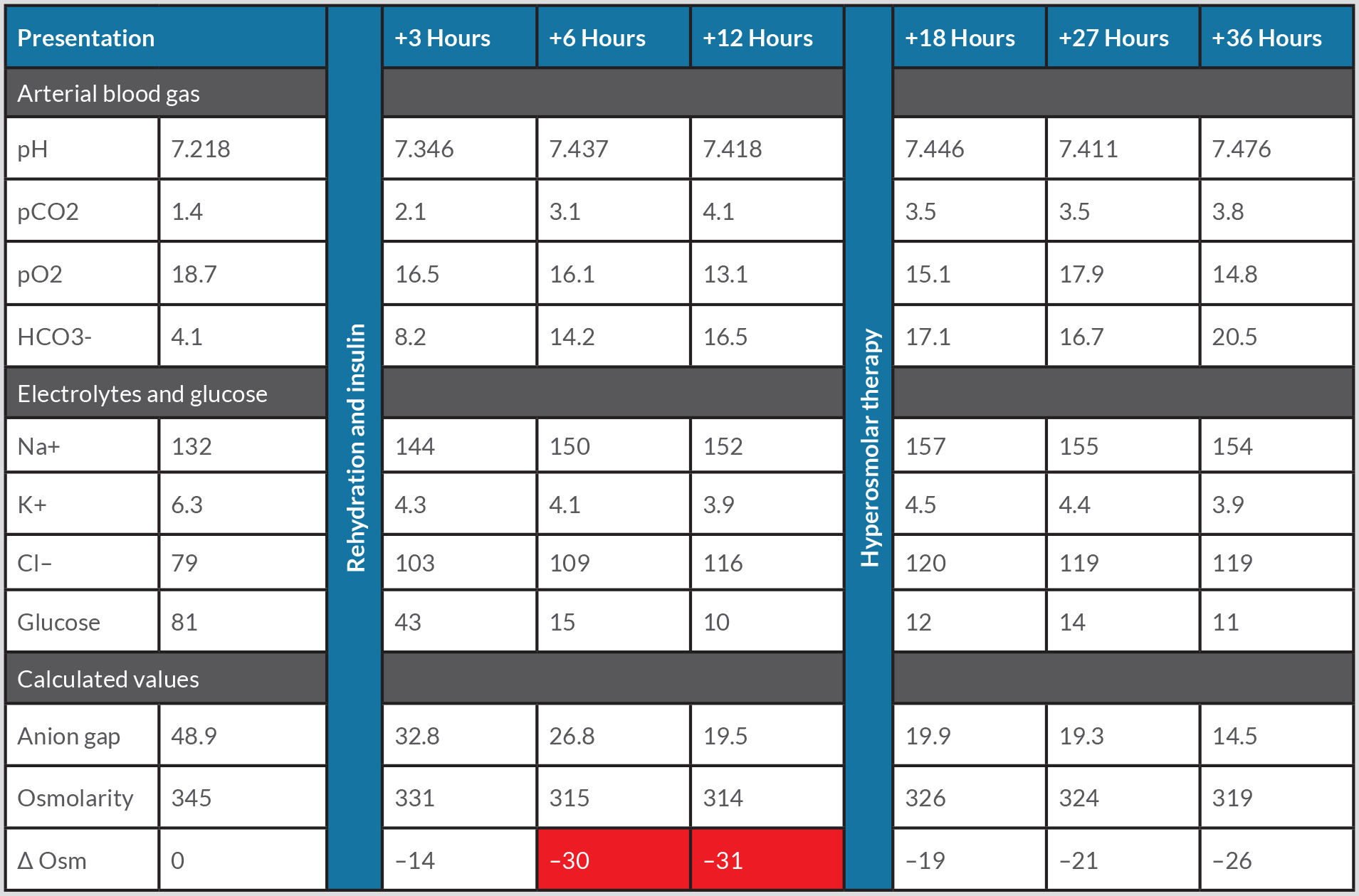
Blood chemistry revealed severe hyperglycaemia of 81 mmol/l, with an effective osmolarity of 345 mOsm/l. Haemoglobin was 11.2 mmol/l, whereas HbA1c was 12.6%. Arterial blood gas analysis showed a pH of 7.22, pCO2 1.4 kPa, pO2 18.7 kPa, and HCO3 4.1 mmol/l. The creatinine level was 275.8 µmol/l. Ketone bodies and glucose were strongly positive in the urine. Table 1 shows the course of the biochemical values obtained during the first 36 hours.
Table 1. Patient’s biochemical values during the first 36 hours of admission, after initiation of treatment, and after hyperosmolar therapy.
Gas values in kPa; electrolytes in mmol/l; Δ Osm, difference in effective osmolarity in mOsm/l; Δ, change in value. Red shading indicates a decrease beyond acceptable limits.
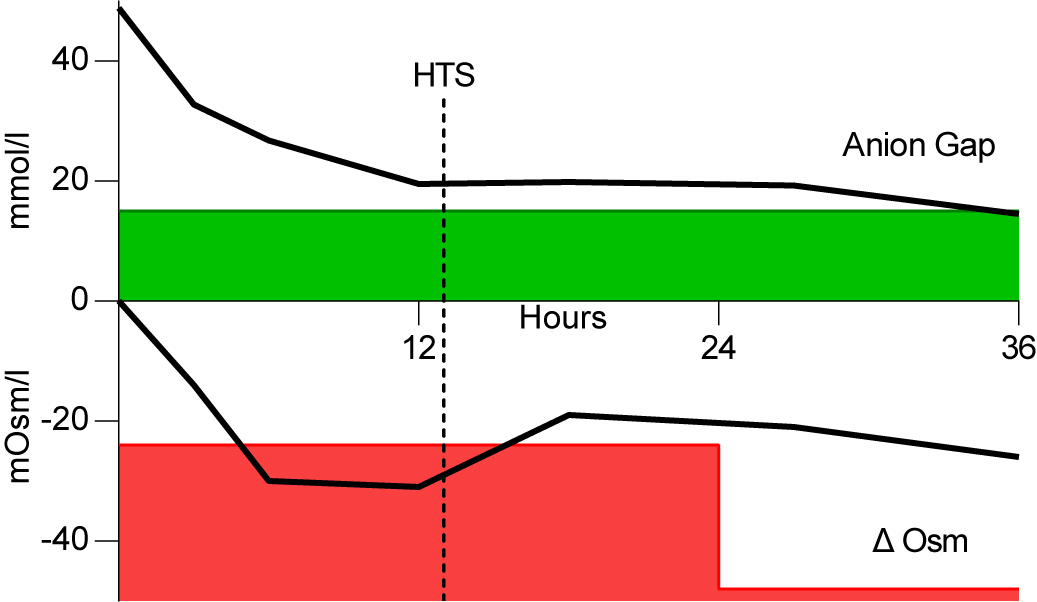
The patient was admitted to the intensive care unit with combined HHS and DKA based on insufficient insulin usage. In adherence to the recommended initial management by the American Diabetes Association (ADA), the patient was treated with an insulin bolus of 0.1 U/kg i.v., followed by 0.1 U/kg/h and normal saline at 500–1000 ml/h for the first 2–4 hours. After the initial fluid replenishment, saline infusion was continued at a rate of 4000 ml per 24 hours. This resulted in an osmolarity decrease of 13.5 mOsm/l in the first 3 hours and 30 mOsm/l within 6 hours. This was accompanied by patient drowsiness. We expected a further osmolarity decrease beyond safe limits and so started hyperosmolar therapy after 13 hours with NaCl 3% at a rate of 200 ml/h. As depicted in Fig. 1, the osmolarity increased 12 points to a safer range at 18 hours after presentation and thereafter decreased more gradually. Subsequently a switch to only isotonic fluids and subcutaneous insulin could be made. The patient recovered and was discharged.
Figure 1. Course of anion gap and effective osmolarity during the first 36 hours. X-axis in hours, Y-axis in mmol/l or mOsm/l for anion gap and effective osmolarity, respectively. Green shading indicates an acceptable anion gap (<15); red shading indicates the unacceptable change in effective osmolarity; HTS, hypertonic saline (NaCl 3%); Δ Osm, difference in effective osmolarity.
It should be noted that cerebral adaptive mechanisms allowing osmolar change over time do not follow an intermittent stepwise decrement every 24 hours as depicted by the green shading, but this serves as a convenient illustration of treatment limits.
DISCUSSION
A recent retrospective study of 1211 patients found that about 27% of patients presented with combined features of HHS and DKA and that this combination resulted in a two-fold increased (odds ratio 2.7) mortality risk compared with isolated diabetic emergencies [1]. Therefore, it is noteworthy that no prospective studies have determined the best treatment strategies for the management of coexisting HHS and DKA. However, awareness of the pathophysiology of these diabetic complications can guide treatment decisions [2].
DKA develops when absolute insulin deficiency results in loss of lipolysis inhibition. The resulting fatty acid metabolism causes ketone production with acidaemia, the hallmark of DKA. In relative insulin deficiency, there is sufficient insulin to prevent lipolysis, but insufficient for appropriate utilization of glucose, causing its serum concentration to increase gradually. The increase in serum osmolarity draws free water out of the extravascular space that is subsequently lost through diuresis together with glucose and electrolytes causing severe dehydration, the hallmark of HHS. This volume-depleting mechanism occurs in both diabetic emergencies, but to a different degree; the total body water deficit is approximately 6 litres in DKA and 9 litres in HHS. This problem causes renal impairment and a (dilutional) hyponatraemia in both, as water is osmotically shifted from the intracellular to the extracellular space [2].
Both syndromes require volume repletion and insulin, however, the different pathogeneses demand different treatment priorities and considerations. Rapid changes in serum osmolarity during treatment might precipitate cerebral oedema [3]. With insulin treatment for DKA, the decrease in serum osmolarity is often gradual; the decrease in serum glucose resolves (dilutional) hyponatremia, protecting the brain from further hazardous osmotic shifts. In HHS, however, extremely high levels of glucose may increase the risk of an overly rapid correction of osmolality if insulin is started at presentation. The combination of HHS and DKA therefore leads to a treatment dilemma because both fluid repletion and insulin therapy may be required initially.
The Joint British Diabetes Societies (JBDS) for Inpatient Care recommends treating HHS without DKA with fluid repletion and delaying insulin therapy until blood glucose levels are no longer falling with fluid therapy alone. The proposed reduction of osmolarity by 3–8 mOsm/l/h equates to an accepted osmolarity reduction of up to 2 mOsm/l/h. The JBDS states that in concurrent significant ketosis and acidosis, insulin should be started and usage of the guideline should be modified, but no recommendations concerning the limits of osmolality decrease are given [4]. The ADA advocates immediate treatment through rehydration and insulin for HHS as well as DKA, and recommends prevention of cerebral oedema through ‘avoidance of rapid reduction in serum osmolarity’, but interestingly, the preferred rate of reduction is not specified [2].
In patients with hypernatraemia without diabetes, there is a general consensus that sodium correction should not exceed 12 mEq/l/day [4]. In the effective osmolarity formula, 2×(sodium+potassium)+glucose, this equates to a maximum decrease in osmolarity of 24 mOsm/l/day, or 1 mOsm/l/h.
As shown in Fig. 1, in this case, the rate of osmolarity reduction remained within the JDBS recommendation but exceeded the safe limits for the hypernatraemia-based osmolarity reduction rate. The standard therapy may treat hyperosmolarity and ketoacidosis state but cause a dangerous decrease in osmolarity. We postulated that the administration of hyperosmolar therapy could counterbalance the decrease in effective osmolarity without compromising insulin suppletion. We initiated hyperosmolar sodium infusion (NaCl 3%) to induce further hypernatraemia and, as demonstrated in Fig. 1, we were able to continue insulin and simultaneously halt the excessively decreasing osmolarity.
Prospective studies for the management of HHS and DKA are necessary, but in the meantime it seems prudent to avoid large decreases in osmolarity during the first days of treatment. Although saline 3% may be an option to control the decline in osmolarity, its use is not without risk. No guidelines are established for its use in HHS and DKA and as a 3% solution has a sodium content of 513 mEq/l, it has to be used with caution in patients with hypertension, heart failure, cirrhosis, renal impairment and oedema. In addition, electrolyte imbalances, a normal anion gap metabolic acidosis, and a prolonged hyperosmolar state may be induced. The use of hypertonic saline should be guided by frequent electrolyte and osmolarity monitoring and the decrease in osmolarity should be probably not exceed 1 mOsm/l/hour for the first 48 hours.
.