ABSTRACT
Bullous pemphigoid is a rare autoimmune dermatologic disease that usually occurs in the elderly. Mucous membrane lesions occur in about 10–35% of patients and are almost always limited to the oral mucous membrane. Esophageal involvement is very rare (4% of cases) and usually presents with chest pain, dysphagia, and odynophagia, though patients are frequently asymptomatic. We report the case of newly diagnosed bullous pemphigoid in a 76-year-old man with a past medical history of dementia. He presented with cutaneous manifestations but also severe gastrointestinal bleeding due to extensive esophageal involvement. Although bullous pemphigoid is mainly a skin disease, mucous membrane lesions should not be overlooked as they are associated with an even poorer outcome. A high index of suspicion for esophageal involvement is needed as its presentation can be fatal, as with our patient.
LEARNING POINTS
- Bullous pemphigoid is a rare autoimmune disease that should be suspected in elderly patients with itchy cutaneous lesions.
- Mucous membrane lesions should always be evaluated, as they are associated with a poor prognosis, even if asymptomatic.
- Early diagnosis should be the main focus, as steroids, the mainstay of treatment, may not be effective in severe cases.
KEYWORDS
Bullous pemphigoid, esophagus, dementia
CASE DESCRIPTION
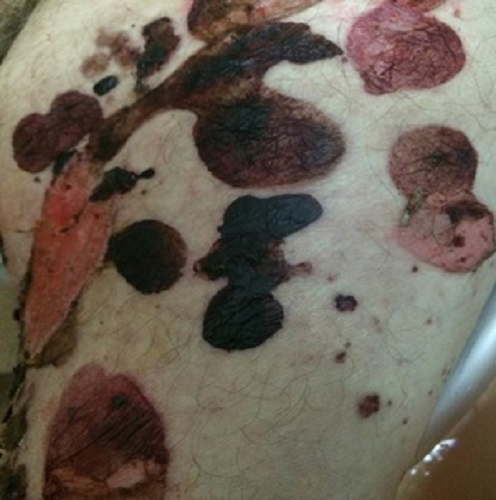
We report the case of a 76-year-old man with a 4-year history of dementia who presented to the emergency department in our hospital with a 3-month history of cutaneous blisters. Physical examination revealed widespread tense bullae in the trunk, abdomen, and limbs but with no oral lesions (Fig. 1). He was admitted to the ward and evaluated by the dermatologic team: a skin biopsy was performed, blood analysis undertaken and he was started on prednisolone 1 mg/kg.
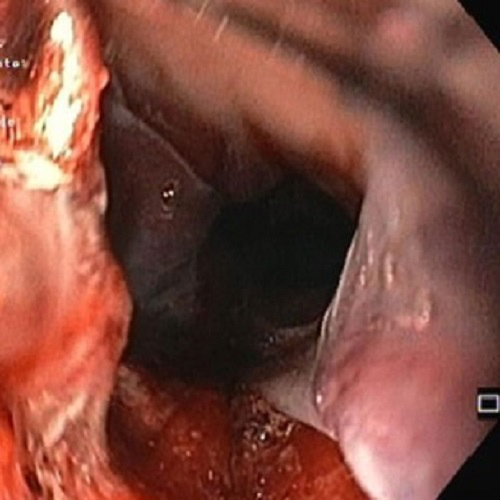
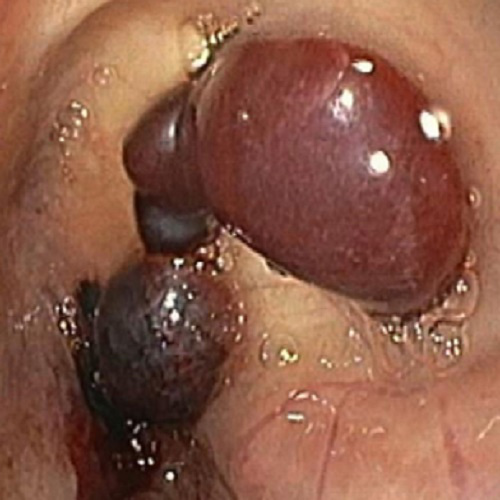
On day 2 of his hospital stay, he developed melena and hematemesis, with a resulting decrease in hemoglobin from 10.2 g/dL to 9.2 g/dL. An urgent upper endoscopy was performed that showed numerous blistering lesions on the hypopharynx (Fig. 2) and a large bleeding esophageal hematoma as well as active bleeding of the esophagus (Fig. 3). Despite intensive treatment with methylprednisolone and epsicaprom at the time, the patient died from hemorrhagic shock the following day. The previously obtained blood analysis showed antibodies against bullous pemphigoid (BP) antigen180 and a high titer of anti-basement membrane antibodies. The skin biopsy had identified subepidermal blisters with numerous eosinophils and a superficial dermal inflammatory cell infiltrate. A diagnosis of BP was subsequently made.
Figure 1. Bullous pemphigoid showing skin lesions
Figure 2. Blistering lesions on the hypopharynx
Figure 3. Large bleeding esophageal hematoma
DISCUSSION
BP is the most common autoimmune blistering disorder, with 2.5–42.8 cases diagnosed per million per year[1]. It is far more common in the elderly, mainly in the eighth decade of life, and its incidence has increased in recent years[2]. A review by Lai et al. showed a close association between BP and neurologic diseases, mainly dementia, Parkinson’s disease, stroke, epilepsy, and multiple sclerosis, with a five-time higher risk, where BP usually develops 5.5 years after the onset of neurologic disease[3]. Cases have also been reported of a higher risk of BP in patients with hematologic malignancies[1].
BP usually presents with tense blisters on normal skin or erythematous lesions mainly in the axillary folds, lower abdomen, inner thighs, and inguinal areas. Mucous membrane lesions occur in up to 35% of cases, but pharynx and esophageal involvements are rare, especially with no accompanying oral lesions. Esophageal involvement may be asymptomatic or present with chest pain, dysphagia, and odynophagia, while upper endoscopy can show bullous and necrotic areas[4]. The diagnosis should be suspected in elderly patients with itchy cutaneous lesions and clinical evaluation should include a meticulous dermatologic examination. Diagnosis is made by direct immunofluorescence of the lesions, which shows linear deposition of IgG and/or C3 along the basement membrane zone and quantification of serum anti-BP180 and anti-BP230 by ELISA (enzyme-linked immunosorbent assay)[1,5]. The differential diagnoses should include pemphigus foliaceus, dermatitis herpetiformis, epidermolysis bullosa or bullous lupus erythematosus[1]. Systemic steroids are the mainstay of treatment (prednisone or prednisolone), although there are some successful reports of treatment with azathioprine or rituximab[6,7]. More recently, omalizumab, a monoclonal anti-IgE antibody, has emerged as a possible treatment option[1].
The prognosis of BP is poor, with a 1-year combined mortality rate of 23%, while older age, neurological disorders, and higher serum levels of anti-BP180 aggravate the risk of a fatal outcome[8,9].