ABSTRACT
Hepatic encephalopathy is a common complication in chronic liver disease and cirrhosis. Here we describe two patients with hepatic encephalopathy who did not respond to standard empiric treatment and were found to have non-convulsive status epilepticus. Both patients improved with antiepileptic therapy. Non-convulsive status epilepticus should be considered in the differential diagnosis of patients with suspected hepatic encephalopathy who do not respond to empiric treatment.
LEARNING POINTS
- Non-convulsive status epilepticus (NCSE) is a rare complication of hepatic encephalopathy (HE).
- Clinical evaluation should be used to rule out different causes of altered mental status in patients with chronic liver disease.
- Consider EEG to diagnose NCSE in patients with suspected HE not responding to empiric treatment.
KEYWORDS
Hepatic encephalopathy, non-convulsive status epilepticus, electroencephalogram
INTRODUCTION
In the USA, more than 4.5 million people have chronic liver disease [1]. Some 30–45% of patients with cirrhosis can develop hepatic encephalopathy (HE) leading to significant morbidity and mortality [2, 3]. The clinical spectrum of HE extends from mild cognitive impairment to coma [4]. Non-convulsive status epilepticus (NCSE) is a state of ongoing seizure activity for at least 30 min without convulsive clinical manifestations. It requires an electroencephalogram (EEG) for confirmation. Due to similarities in the clinical spectrum of HE and NCSE, the latter, more morbid and uncommon diagnosis can be missed [5, 6]. Here we present two patients with liver cirrhosis without a previous history of seizures, who presented with HE and were diagnosed with NCSE.
CASE DESCRIPTIONs
Case 1
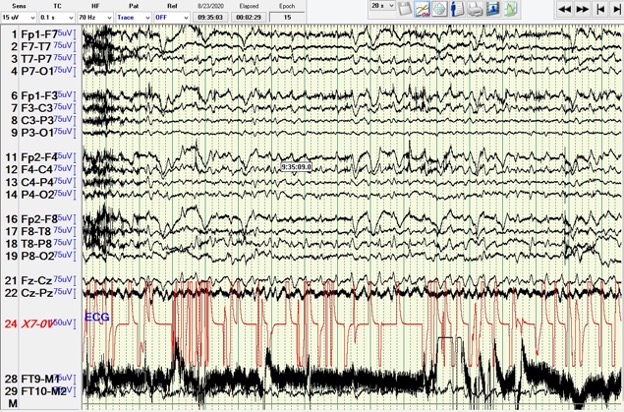
A 47-year-old woman with a medical history of class III obesity, alcohol use disorder with dependence, and upper gastrointestinal bleed presented with a 4-day history of confusion, falls and jaundice. She was never formally diagnosed with cirrhosis. Somnolence, dilated pupils, scleral icterus, distended abdomen, +2 pitting oedema in the legs, and asterixis were present on examination. Leucocytosis, anaemia, hyponatremia and deranged liver function tests were present on initial evaluation. Liver ultrasound showed hepatomegaly with mild ascites. The patient was diagnosed with HE and alcoholic hepatitis. On admission, she had an acute kidney injury. Lactulose was commenced. Due to lack of symptomatic improvement, rifaximin was added. Her last alcoholic drink was about 3 weeks before presentation, ruling out alcohol withdrawal. On the 6th hospital day, her mental status improved and was normal for 3 days before deteriorating on the 9th day. She had an adequate response to lactulose and rifaximin with three to five bowel movements daily. Her kidney injury further worsened and she was diagnosed with hepatorenal syndrome with an albumin challenge test. Due to lack of response, EEG was ordered. She was found to be in NCSE (Fig. 1). She was given stat intravenous lorazepam, levetiracetam, and transferred to the intensive care unit, resolving her NCSE. Lactulose, rifaximin and tube feeds via a nasogastric tube were continued. Her course was complicated with aspiration pneumonia. Multiple repeat EEG were negative for seizure activity and she was maintained on levetiracetam. Haemodialysis was also started due to persistent oliguria before subsequent discharge to a skilled nursing facility.
Case 2
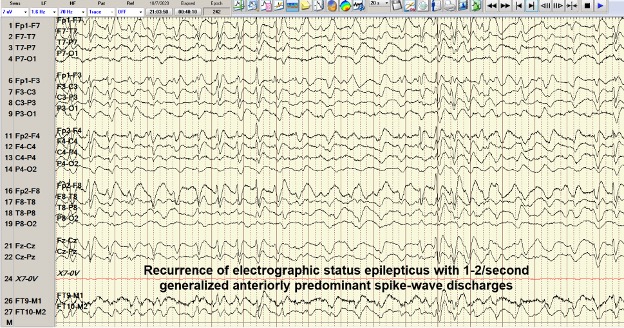
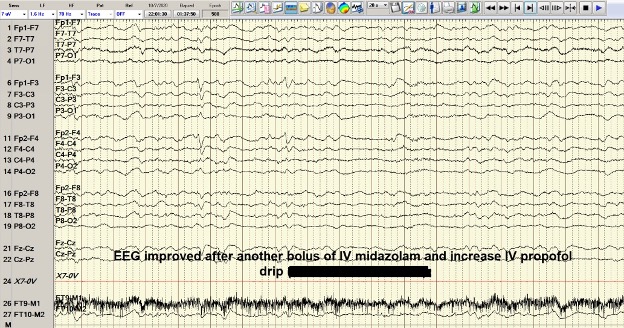
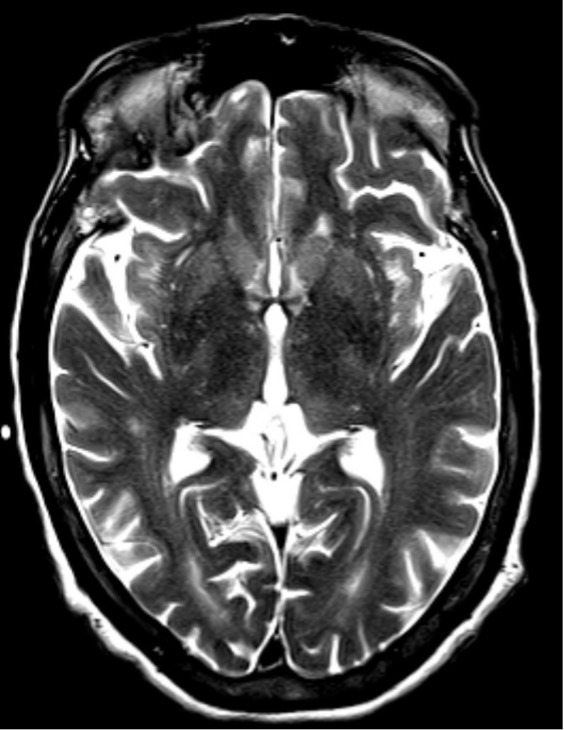
A 53-year-old man with a history of hepatitis C, hepatitis B, liver cirrhosis, upper gastrointestinal bleed, and intravenous drug use, presented with one episode of haematemesis and altered sensorium. He was not actively drinking and was compliant with his lactulose. His ammonia level was 323 and he was started on rectal enema. AST, ALP and INR levels were elevated. Albumin levels were low. Somnolence, abdominal distension and asterixis were noted. He was intubated for airway protection. During endoscopy, large, active varices found in the lower third of the oesophagus, were banded. Afterwards, his electrocardiogram showed ST-segment elevations. Cardiac catheterization revealed non-obstructive coronary artery disease. He continued to be obtunded on the 2nd hospital day so an EEG was ordered that showed NCSE (Fig. 2). He was successfully treated with intravenous midazolam bolus, levetiracetam load and propofol (Fig. 3). Magnetic resonance imaging of the brain revealed hyperintense signals in the bilateral medial thalami and putamen (Fig. 4). On the 5th hospital day, he had transient left-sided facial droop and left-sided weakness. Repeat imaging studies were unremarkable. He was discharged to a skilled nursing facility and levetiracetam was discontinued.
Figure 2. Case 2: EEG showing status epilepticus
Figure 3. Case 2: EEG showing status epilepticus after burst suppression
Figure 4. Case 2: MRI of the brain without contrast. Axial section of T2 series showing hyper-intense signal in the bilateral medial thalami and putamen
DISCUSSION
HE, characterized by altered mental status, occurs in chronic liver disease. The multifactorial pathophysiology has many culprit agents including cytokines, cerebral oedema, and intracranial hypertension [7, 8]. Ammonia, a major culprit, cannot be metabolized in patients with chronic liver disease and is, therefore, shunted to the systemic circulation without detoxification [9]. The management of HE involvesreduction of either ammonia production and absorption [10].
The pathophysiology of seizures in HE remains unknown. Elevated ammonia results in increased glutamine production that promotes generalized swelling leading to neuronal dysfunction [11, 12]. The permeability of the blood–brain barrier for ammonia increases during inflammation, enhancing toxicity [12]. The epileptiform activity in EEG, seizures or status epilepticus (SE), generally imply a poor prognosis because they develop in end-stage liver disease [6, 13].
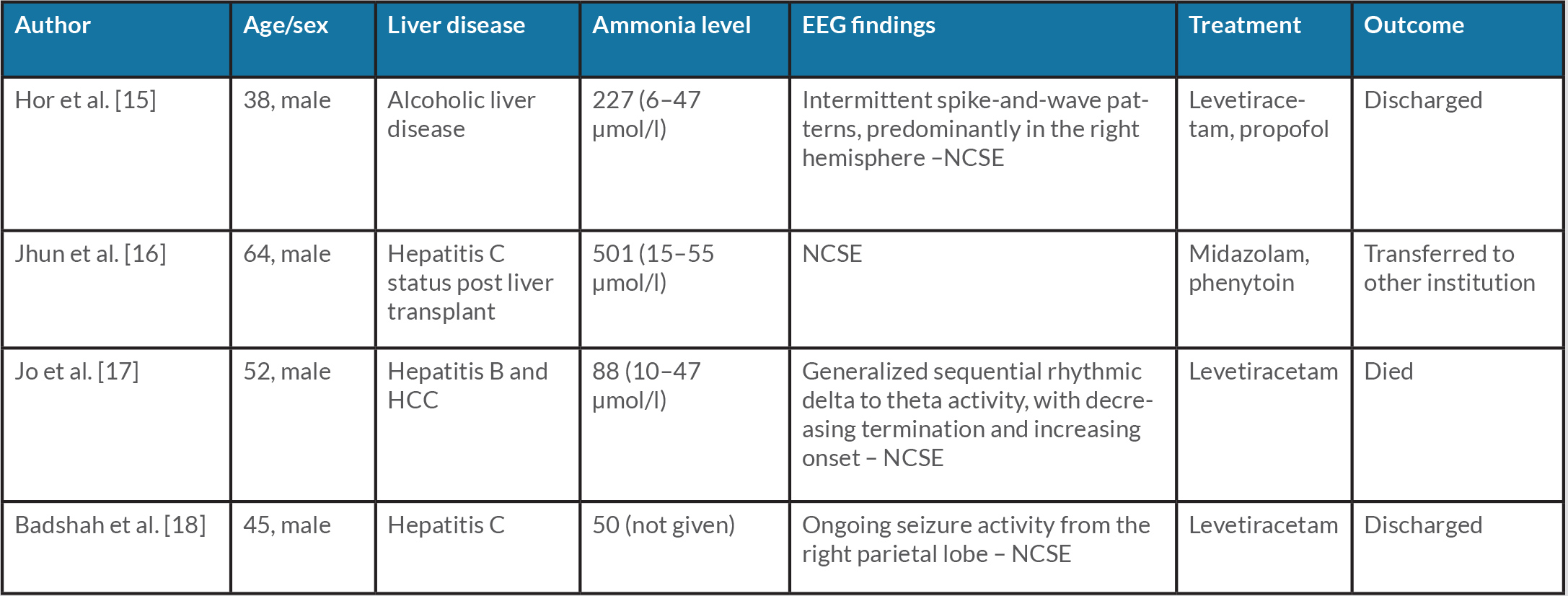
In our study, neither patient responded to ammonia-reducing treatment and both were diagnosed with NCSE on EEG. With proper treatment, their mentation improved and they were discharged. A high level of clinical suspicion is required to identify NCSE that is associated with elevated mortality [5]. Gursky et al. showed that HE events were associated with subsequent admissions for epilepsy or SE [14]. This effect was even higher for admission with SE, with a three- to fivefold likelihood of admission with SE in the next 6 months [14]. Table 1 summarizes the four case reports found in PubMed, CINAHL and Web of Science [15–18].
Table 1. Summary of articles found in databases
EEG, electroencephalogram; HCC, hepatocellular carcinoma; NCSE, non-convulsive status epilepticus.
CONCLUSION
NCSE should be considered in the differential diagnosis of patients with HE who do not respond to the usual treatment. Further studies are needed to investigate NCSE.