ABSTRACT
Introduction: The detection of pneumococcal antigens in urine is an alternative to gram staining, and their culture is central to the diagnosis of pneumococcal pneumonia. We present a case of the false-positive detection of urinary Streptococcusspecies pneumococcal antigen with a BinaxNOW test. This resulted in delayed diagnosis of a liver abscess.
Case description: A 70-year-old woman presented to the emergency department with a 1-day history of chills and difficulty walking. She had a fever and her physical examination was normal. Non-contrast chest computed tomography (CT) revealed a slight ground-glass opacity in the left lower lobe. Laboratory tests revealed liver injury and elevated C-reactive protein levels. A urinary pneumococcal antigen test was positive, and she was diagnosed with acute bronchopneumonia caused byStreptococcus pneumoniae. She was treated with ceftriaxone. However, abdominal contrast-enhanced CT performed the next day revealed portal vein thrombus and a left lobe liver abscess. Streptococcus constellatus was detected in a puncture specimen of the liver abscess. It was concluded that the positive urinary pneumococcal antigen test was a false-positive owing to Streptococcus infection.
Discussion: False-positive results might be explained by the presence of C-polysaccharide antigens in the cell wall of S. pneumoniae. The positive urinary antigen test together with the finding of slight ground-glass opacity in the left lung on chest CT initially led to misdiagnosis. False positives may result in misdiagnosis and unnecessary antimicrobial therapy.
Conclusion: The overuse of the pneumococcal urinary antigen tests can lead to false positives and misdiagnosis.
LEARNING POINTS
- False-positive pneumococcal urinary antigen results may lead to the misdiagnosis of pneumococcal pneumonia caused byStreptococcus pneumoniae and unnecessary antimicrobial therapy.
- False-positive results can occur in patients with infections caused by other Streptococcus species (e.g., liver abscess caused by Streptococcus constellatus).
KEYWORDS
Pneumococcal urinary antigen, pneumococcal pneumonia, Streptococcus, liver abscess, false-positive, misdiagnosis
CASE DESCRIPTION
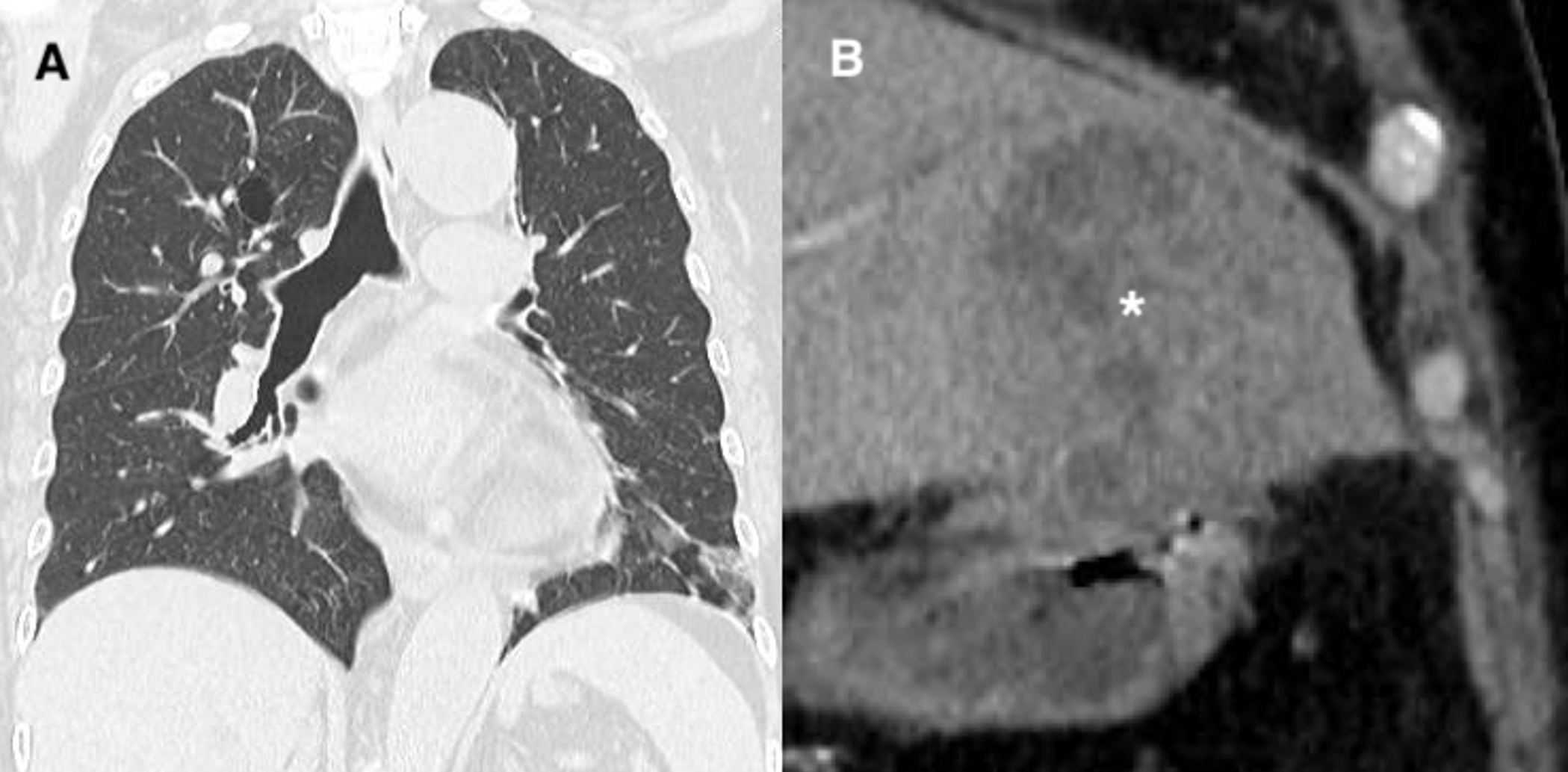
A 70-year-old woman presented to the emergency department (ED) with a 1-day history of chills and difficulty walking. She had attended her primary care physician after developing fatigue and had been prescribed cefdinir with no improvement. Her vital signs in the ED were: blood pressure 100/60 mmHg, temperature 38.6°C, pulse rate 120 beats per minute, respiratory rate 28 breaths per minute, and oxygen (O2) saturation 94% on room air. On physical examination, her lungs were clear to auscultation, and the abdominal examination was unremarkable. Non-contrast chest computed tomography (CT) revealed a slight ground-glass opacity in the lower lobe of the left lung (Fig. 1A). The laboratory test results were as follows: white blood cell count 5900/µl (normal range: 3300–8600/µl), haemoglobin level 11.3 g/dl (normal range: 11.6–14.8 g/dl), platelets 24.9×104/µl (normal range: 15.8–34.8×104/µl), serum levels of total bilirubin 0.6 mg/dl (normal range: 0.4–1.5 mg/dl), direct bilirubin 0.4 mg/dl (normal range: <0.3 mg/dl), aspartate aminotransferase 51 U/l (normal range: 13–30 U/l), alanine aminotransferase 67 U/l (normal range: 7–23 U/l), lactate dehydrogenase 386 U/l (normal range: 124–222 U/l), alkaline phosphatase 530 U/l (normal range: 106–322 U/l), γ-glutamyl transpeptidase 113 U/l (normal range: 9–32 U/l), blood urea nitrogen 14.9 mg/dl (normal range: 8–20 mg/dl), creatinine, 0.91 mg/dl (normal range: 0.53–1.02 mg/dl), and C-reactive protein 221.4 mg/l (normal range: 0–1.4 mg/l). A BinaxNOW test to assess pneumococcal antigen was positive. Thus, the patient was diagnosed with acute pneumonia caused byStreptococcus pneumoniae and treated with ceftriaxone.
The next day, the attending physician, on assessing the chest CT findings, determined that the elevated levels of liver and biliary system enzymes were unlikely to be attributable to pneumonia alone. A subsequent abdominal contrast CT revealed a thrombus in the left branch of the portal vein and a liver abscess with a maximum diameter of 8 cm in the left liver lobe (Fig. 1B). Streptococcus constellatus, Proteus mirabilis and Bacteroides fragilis were detected in a liver abscess puncture specimen. The patient had not recently had pneumonia or pneumococcal vaccination. We determined the positive urinary pneumococcal antigen finding was a false-positive result owing to infection with a different Streptococcusspecies. The patient received percutaneous drainage on the second day of hospitalization and was discharged without complications on day 18.
Figure 1 (A) Slight ground-glass opacity in the lower lobe of the left lung on non-contrast computed tomography (CT). (B) Abscess in the left lobe of the liver (*) on abdominal contrast-enhanced CT
DISCUSSION
Pneumococcal pneumonia caused by the bacterium S. pneumoniae is a serious illness warranting immediate treatment and hospitalization. A definitive diagnosis can be obtained by isolating causative bacteria from the blood or pleural fluid [1]. A positive gram stain and culture of high-quality sputum samples provide strong evidence for pneumococcal pneumonia. However, culture has high rates of negative results [2].
An alternative method to culture sampling is the detection of pneumococcal antigens in urine. Although this test is rapid and easy to perform, recent studies have raised questions regarding its accuracy, clinical use and cost-effectiveness [1]. Further, routine use of urinary antigen tests for detecting community-acquired pneumonia is uncommon [1]. The most frequently used urinary pneumococcal antigen tests in Denmark are ImmuView (S. pneumoniae and Legionella pneumophila urinary antigen test; SSI Diagnostica, Hillerød, Denmark) and BinaxNOW (S. pneumoniae antigen card; Abbott, Denmark). There have been reports of false-positive urinary pneumococcal antigen test results [3–5], but little is known about their impact on the diagnosis of pneumococcal pneumonia. The urinary antigen test for S. pneumoniae has a reported sensitivity, specificity, positive likelihood ratio and negative likelihood ratio of 71–82%, 96–97%, 17–28 and 0.19–0.3, respectively, with high diagnostic accuracy [6, 7]. The antigen test has various advantages over sputum culture including convenience, the ability to test patients who are unable to expectorate sputum, and providing results that are unaffected by antimicrobial use [2].
However, the use of urinary antigen tests is controversial, with some reports indicating that they are useful for the prescription of appropriate antimicrobial agents [7], while others suggest that they did not improve clinical outcomes. Further, antimicrobial prescription and a narrow treatment range based on the urinary antigen test results increase the risk of clinical relapse [1]. Consequently, the 2019 Infectious Diseases Society of America/American Thoracic Society guidelines do not recommend the routine use of urinary antigen tests in non-critically ill patients [8]. In contrast, in their the 2017 guidelines for community-acquired pneumonia, the Japanese Society of Respiratory Medicine weakly recommend urinary antigen testing for all patients [9].
False-positive pneumococcal urinary antigen test results have been reported in carriers of S. pneumoniae, and patients with a history of previous pneumococcal vaccination, infections caused by other Streptococcusspecies, and episodes of pneumococcal pneumonia over several months [3–5]. False-positive results due to Streptococcus species can possibly be explained by the presence of C-polysaccharide antigens in the cell wall of S. pneumoniae [3].
There are no case reports of false-positive urinary pneumococcal antigen detection being associated with delayed diagnosis. Although our patient was ultimately diagnosed with a liver abscess, the positive urinary antigen result along with the observation of a slight ground-glass opacity in the lower lobe of the left lung on chest CT initially led to misdiagnosis.
The results of pneumococcal urinary antigen testing have a low impact on the management of pneumonia and do not affect the cost of treatment [1]. The use of a pneumococcal urinary antigen test at a stage when the diagnosis of pneumonia is unclear—as seen in this case—is an overuse of the test. Further, false positives may lead to misdiagnosis and unnecessary antimicrobial therapy. The optimal use of pneumococcal urinary antigen tests needs to be investigated in the future and evaluated in clinical practice.
CONCLUSION
The overuse of pneumococcal urinary antigen tests can lead to false positives, resulting in misdiagnosis, so their optimal use warrants careful evaluation in practice.