ABSTRACT
Small bowel tumours are rare, representing about 0.5% of all tumours and about 3% of gastrointestinal tract tumours . The low prevalence contrasts with the vast surface area of the small intestine, which accounts for over 90% of the surface area of the digestive tract. The frequency of small tumours decreases from proximal to distal, and therefore from the duodenum to the ileum. The histological types differ in terms of prevalence according to the affected segment, with adenocarcinoma being the most frequent in the duodenum and jejunum and carcinoid tumour in the ileum. Diagnosis is challenging due to clinical non-specificity, low prevalence and a low level of suspicion.
Schwannomas are typically benign tumours that arise from Schwann’s cells and are rarely found in the small intestine. It is even more rare to find them together with another histological type, namely adenocarcinoma. No cases have been reported in the literature of these lesions occurring in the small intestine simultaneously. Further studies are needed to clarify the underlying pathophysiology of these synchronous tumours.
The authors present the case of an 86-year-old female patient admitted for high intestinal subocclusion, with refractory vomiting and involuntary weight loss. Two synchronous lesions in the digestive tract were identified: an adenocarcinoma in the duodenum and a schwannoma in the ileum. The patient underwent surgical resection of both lesions.
A high level of suspicion combined with a multidisciplinary approach is necessary for timely diagnosis and surgical resolution.
LEARNING POINTS
- Small bowel neoplasms are rare and clinically non-specific; in addition, diagnosis is difficult due to imaging artifacts and tumour inaccessibility for biopsy for definitive histological diagnosis.
- Gastrointestinal schwannomas are rare and the pathophysiology of synchrony with other histological subtypes remains to be clarified.
- A multidisciplinary approach from the beginning is important for a timely diagnosis and better outcome.
KEYWORDS
Small bowel tumours, schwannoma, synchronous lesions
CASE DESCRIPTION
The patient was a 86-year-old woman with a history of hysterectomy for uterine fibroids, Helicobacter pylori gastritis eradicated 10 years previously, and hypertension. She also had a family history of gastric neoplasia.
She was admitted due to a 1-week history of persistent and refractory vomiting, accompanied by an involuntary weight loss of about 23% of her body weight over 1 month. She denied abdominal pain or changes in intestinal transit. Physical examination was unremarkable except for dehydration and mucocutaneous pallor.
Methods and procedures
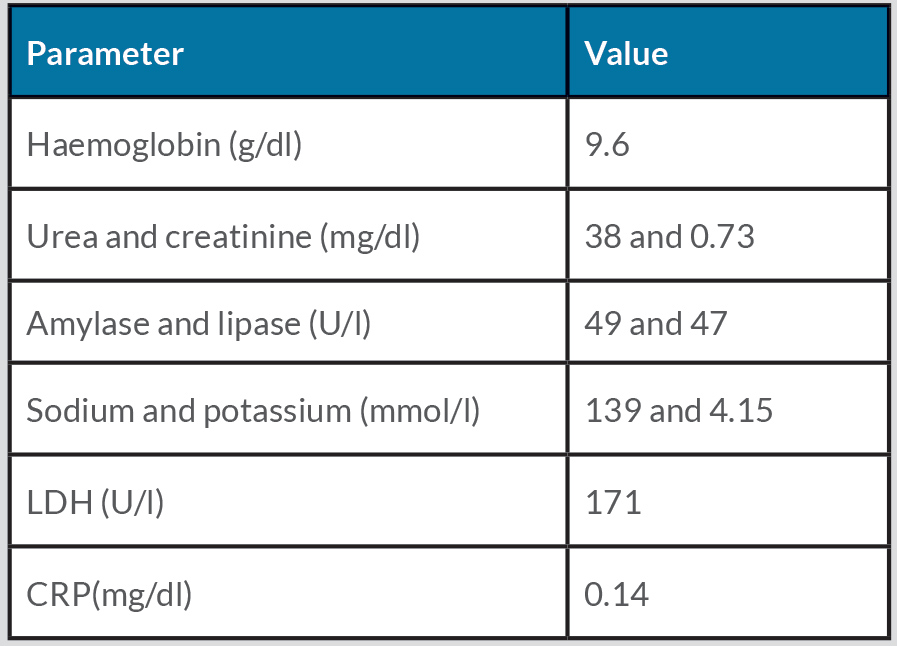
Laboratory tests demonstrated iron deficiency anaemia (haemoglobin 9.6 g/dl), with no other relevant changes (Table 1).
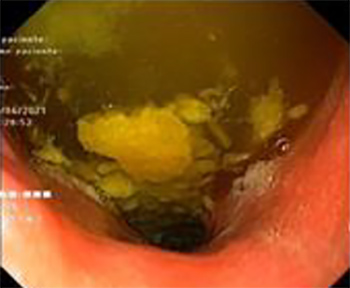
The first upper gastrointestinal endoscopy revealed reflux oesophagitis and abundant food content at the level of the oesophagus, stomach and duodenum (Fig. 1), suggesting a proximal obstruction.
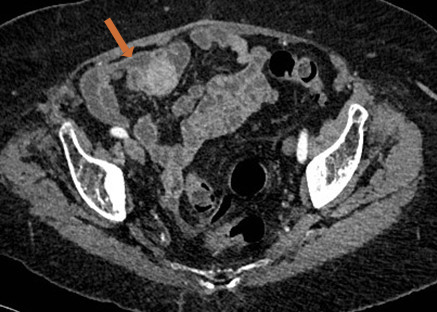
A thoraco-abdominopelvic CT scan revealed an ileal nodular lesion measuring about 2.5 cm, with no signs of invagination or occlusion (Fig. 2).
Table 1. Laboratory evaluation
Figure 1. Food contents (stomach)
Figure 2. Contrast-enhanced abdominal CT (axial section) scan revealing an ileal nodule (arrow)
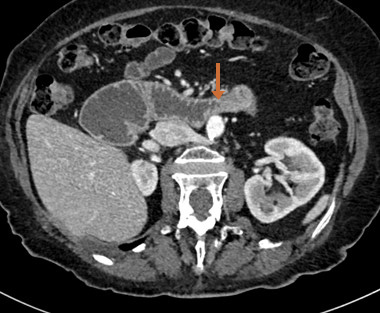
In a multidisciplinary discussion with radiology, there seemed to be a narrowed zone at the level of the third portion of the duodenum with stasis upstream (Fig. 3), and the diagnostic hypothesis of superior mesenteric artery syndrome was raised.
At this stage, a small tumour was assumed, causing significant involuntary weight loss and, consequently, superior mesenteric artery syndrome causing high subocclusion.
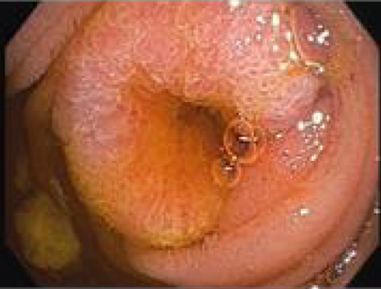
A nasogastric tube was placed to empty the food content and upper digestive endoscopy was repeated, which revealed an insurmountable stenosis neoplasia of the duodenum, at the D3–D4 transition (Fig. 4).
During hospitalization, the patient maintained persistent and refractory vomiting, which led to the institution of parenteral nutrition. In view of the unfavourable clinical evolution and the presence of two synchronous lesions, the patient underwent resection of the small intestine and segmental resection of the ileal lesion. A videocapsule could not be employed due to the risk of retention.
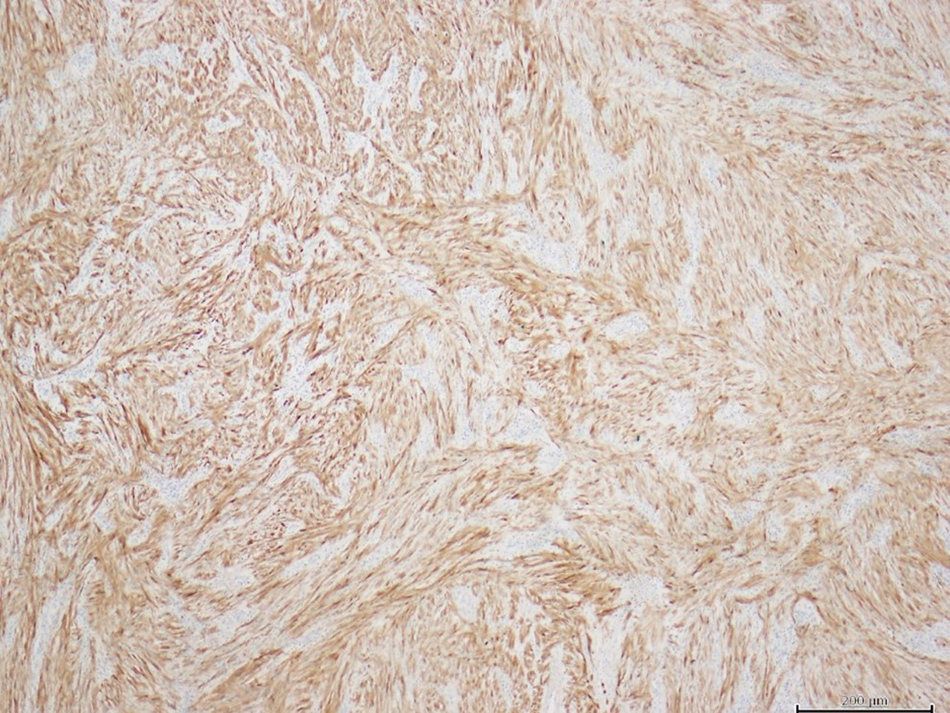
Anatomopathological study of the specimens showed two distinct lesions with different histology: a moderately differentiated duodenal adenocarcinoma and an ileal schwannoma, with cells characteristically expressing PS100 (Fig. 5) and negative for actin, desmin, CD117 and DOG1.
One month after clinical discharge, there was resolution of vomiting and the patient had gained weight. A multidisciplinary meeting was proposed to the patient to discuss possible therapeutic alternatives that might be necessary, but she rejected additional treatment.
Figure 3. CT (axial section) scan revealing a zone of narrowing in D3 (arrow) with stasis upstream
Figure 4. Endoscopy revealing a stenosing lesion between D3 and D4
Figure 5. Ileal schwannoma (expressing PS100)
DISCUSSION
Tumours of the small intestine account for 3.3% of gastrointestinal tumours, 30–40% of which are adenocarcinomas, with a predilection for the duodenum and jejunum [1,2].
Gastrointestinal submucosal schwannomas are rare, originating in Schwann’s cells of the myenteric plexus. They are typically an incidental and benign finding, with a good prognosis after surgical resection. They account for 2–7% of gastrointestinal mesenchymal tumours: 60–70% are found in the stomach and 3% in the colon, but they are rarely reported in the small intestine [3]. The cells express PS100 and are negative for actin, desmin, CD117 and DOG1, differentiating them from gastrointestinal stromal tumours [4]. They present a surgical challenge due to the difficulty of preoperative diagnosis, since clinical, endoscopic and imaging findings are non-specific. The definitive diagnosis is established through histological and immunohistochemical study.
A literature review found only two cases of synchronous lesions of the gastrointestinal tract with these histological subtypes (one in the stomach and the other in the colon), and no cases of synchrony of these lesions in the small intestine [5]. The simultaneous existence of these two types of tumours raises the question whether they result from a simple incidental association or whether they share a common aetiological mechanism. Maiorana et al. raised the hypothesis that, on the basis of synchrony, they are caused by genetic mutations [6]. Other hypotheses suggest that the interaction of a single carcinogenic agent with adjacent tissues induces the development of tumours of different histological types in the same organ [7, 8]. Further studies are needed to clarify the underlying pathophysiology of these synchronous tumours.
The clinical features of neoplasms of the small intestine are non-specific and include intermittent abdominal pain, nausea, vomiting and weight loss. The clinical presentation is usually late with a subocclusive picture [9].
The diagnostic difficulty resides in the silent or non-specific presentation, in the limitations of imaging due to movement artifacts, and the inaccessibility of the non-invasive mass for biopsy and a definitive histological diagnosis.
A high level of suspicion combined with careful imaging is necessary for a timely and correct diagnosis, which requires a multidisciplinary approach and surgical resolution.