ABSTRACT
The Amplatzer septal occluder is one of several percutaneous devices used for the closure of secundum atrial septal defects. The main complications are related to the procedure, with infection being the least common. We present the case of a 67-year-old woman with a secundum atrial septal defect, who, 3 years after repair with an Amplatzer occluder, was admitted with sepsis and bacteraemia following recent hospitalization in an intensive care unit. Transoesophageal echocardiography showed the presence of a mobile echogenic structure in the left atrium suggestive of a vegetation. Few cases of late endocarditis involving the Amplatzer device have been reported, even though partial endothelization is one of the risk factors. There are no guidelines for the prevention, diagnosis or management of this complication.
LEARNING POINTS
- The Amplatzer septal occluder is one of several alternatives for the closure of atrial septal defects and has few related complications, with infection being the least common.
- Device-related endocarditis presents either early (<6 months) or late (>6 months).
- In our case, transoesophageal echocardiography played a key role in the diagnosis of late endocarditis.
KEYWORDS
Late endocarditis, Amplatzer, atrial septal defect, transoesophageal echocardiography
INTRODUCTION
The Amplatzer septal occluder is one of several percutaneous devices used for the closure of secundum atrial septal defects. It is made of nitinol-titanium memory wire mesh infused with polyester patches that facilitate occlusion and endothelialisation [1, 2]. Most of the complications are related to the procedure itself, and include embolization, malposition of the device, cardiac perforation and arrhythmias, with infection being the least common complication (0–1%) [3]. Most associated infections occur in the first 6 months after implantation and are related to the procedure [4]. There are few reported cases of late endocarditis involving this device. We present the case of a patient with transient ischaemic stroke secondary to septic thrombi due to late bacterial endocarditis associated with an Amplatzer device.
CASE DESCRIPTION
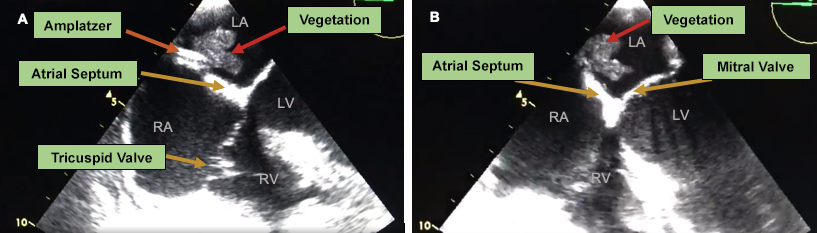
A 67-year-old woman with history of obesity, hypertension, ischaemic stroke and an atrial septal defect (3-mm ostium secundum interatrial communication), which had been percutaneously occluded with an Amplatzer device 3 years previously, was admitted to the emergency department due to 5 days of persistent crampy abdominal pain and vomiting suggestive of intestinal obstruction that required immediate surgery. A right hemicolectomy was performed with an ileocolic anastomosis, revealing a moderately differentiated adenocarcinoma which had spread to nearby lymph nodes and through the peritoneum. She was discharged after 7 days in the intensive care unit in good condition. One week later, the patient attended the emergency room with sudden onset of right-sided weakness that had lasted 3 minutes, with spontaneous recovery. Swelling was evident along the anterior edge of the left sternocleidomastoid measuring approximately 3×4 cm, at the central venous catheter insertion site from the previous hospitalization. Her heart rate was 124 bpm, auscultation revealed sinus tachycardia, and temperature was 39°C. The patient was admitted, and initial laboratory work-up carried out. Blood samples showed leucocytosis and neutrophilia (white cell count 20,120/l, neutrophils 93.8%, lymphocytes 1.3%) and elevated acute phase reactants (PCR 150.7 mg/l). Doppler ultrasonography of neck veins identified deep vein thrombosis in the internal jugular, subclavian and left axillary vein, so anticoagulation with low-molecular-weight heparin (dalteparin 200 U/kg/daily) was initiated in light of her recent neoplasm diagnosis. Blood cultures were obtained which showed methicillin-sensitive Staphylococcus aureus. A transoesophageal echocardiogram performed on hospital day 2 revealed the presence of a large, highly mobile echodense mass on the surface of the Amplatzer device, measuring 19×12 mm with irregular edges and corresponding to a large vegetation, considered to be late endocarditis associated with the device (Fig. 1).
Figure 1. (A,B) Zero-degree 4-chamber view showing an Amplatzer device (orange arrow) located on the atrial septum and a highly mobile hyperechoic image with irregular borders (red arrow) on the left atrial disk, which corresponds to a large vegetation measuring 19×12 mm
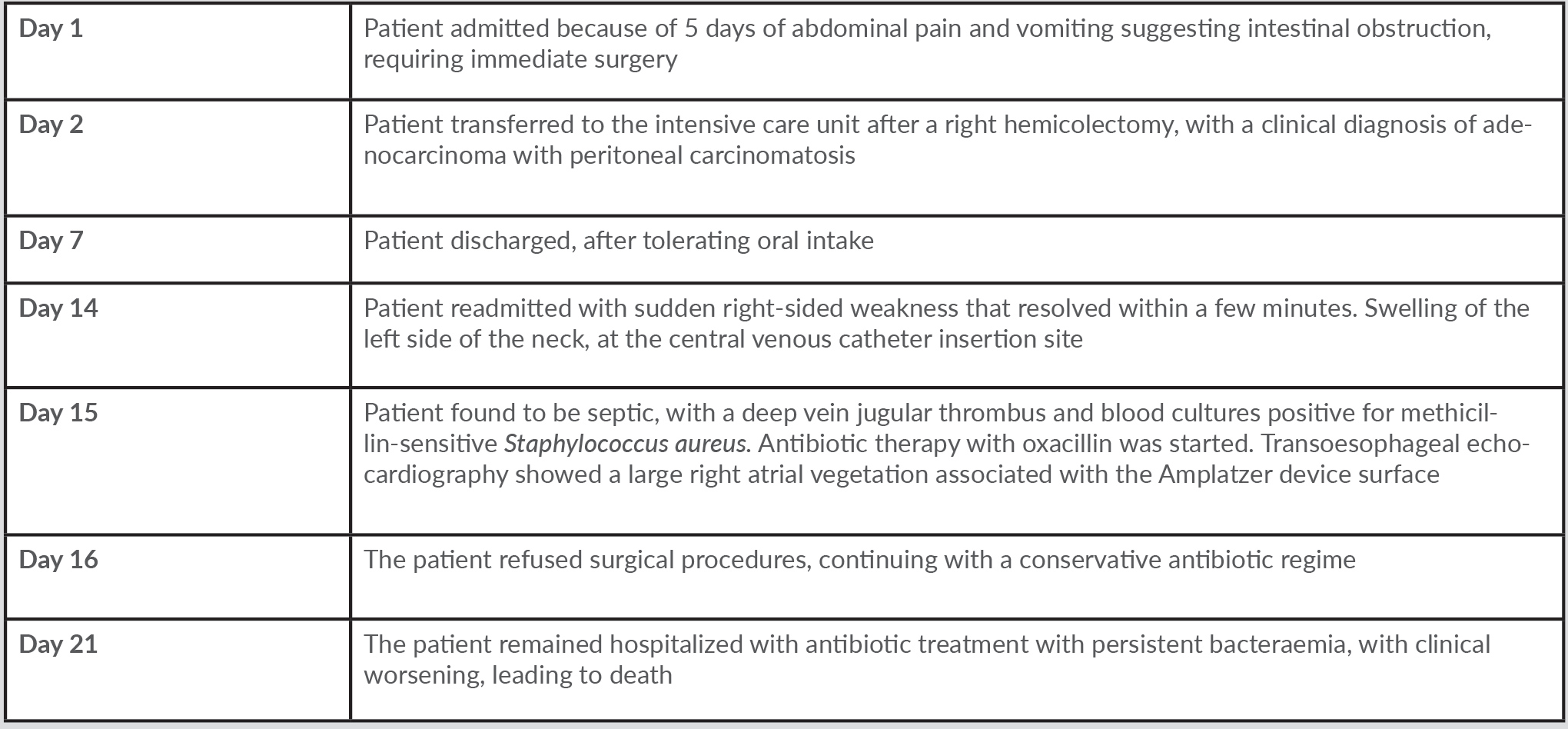
Echocardiographic findings and neurological manifestations suggested a high embolic risk, with the potential need for surgical intervention. However, the patient and her family refused the procedure due to the advanced stage of her recently diagnosed neoplasm, opting instead for a conservative approach with a pathogen-directed intravenous antibiotic regime with oxacillin. The patient remained hospitalized with antibiotic treatment alongside persistent bacteraemia, clinical worsening, increasing dyspnoea, and hypotension, requiring supplemental oxygen and vasopressor, leading to cardiac arrest and death. Table 1 shows the timeline of events.
DISCUSSION
Infection of an Amplatzer device can occur in two ways: in association with the procedure, involving microorganism inoculation, or by later haematogenous spread [4]. Endothelialisation of the device takes 6 months, with the risk of infective endocarditis reducing thereafter[4]. For this reason, the American Heart Association and the European Heart Society recommend 6 months of antibiotic prophylaxis after implantation to prevent early endocarditis [2, 3, 5].
It has been reported that late endocarditis is related to partial endothelialisation, but its actual prevalence is not known [3, 4]. The risk factors for partial endothelization have not been determined either, so there are no current recommendations on the administration of routine prophylaxis to prevent this late complication [3, 4, 6].
Echocardiography has been shown to be an important method for the diagnosis of endocarditis, mainly in cases where the symptoms are not typical and when blood cultures are negative [3, 7]. Transoesophageal echocardiography is substantially better than transthoracic echocardiography for the detection of vegetations, with a positive predictive value of almost 100% [8].
Cerebral embolism is the most common neurological complication of bacterial endocarditis [3]. The most effective way to prevent this occurring is through early diagnosis with the identification of high-risk patients [7]. Echocardiography provides the location and morphological characteristics of the vegetation, so the risk of future complications can be determined and an early surgical approach taken when necessary [1, 8].
There is no consensus on the prevention of late infection which, in our case, presented in a 67-year-old woman 3 years after Amplatzer septal occluder implantation and shortly after ICU hospitalization.
CONCLUSION
Valve prostheses and implanted medical devices are the most common risk factors for infective endocarditis. The literature has described, as in the presented case, the appearance of late endocarditis involving the Amplatzer septal defect occluder device in adults, highlighting the need for surveillance in these patients. Transoesophageal echocardiography allows close anatomical study, with good image quality and resolution, so it should be considered in patients with an atrial septal defect closure device and suspected endocarditis. There are no guidelines on the prevention and management of late endocarditis infection involving the Amplatzer septal defect occluder device.