ABSTRACT
Pulmonary tuberculosis (TB) is a common infection in developing countries and is associated with low socioeconomic status. It is considered a serious infection due it its long-term sequelae. Post-TB lung disease decreases life expectancy and increases the risk of recurrent TB infection. It also significantly increases the risk of other bacterial and fungal infections. Aspergillosis develops in the cavitary lesions of TB, worsening them and resulting in a deteriorating clinical situation. Concomitant pulmonary TB (PTB) and invasive aspergillosis is uncommon, particularly in the absence of factors that suppress the immune system. In this report, we describe the case of a healthy young adult without previous structural lung damage who presented with primary PTB and acute invasive aspergillosis infection, discuss the treatment dilemma and provide a literature review.
LEARNING POINTS
- Aspergillosis can mimic tuberculosis (TB) infection.
- Concomitant pulmonary TB and invasive aspergillosis is uncommon.
- The co-administration of antifungal and anti-TB medications presents significant therapeutic challenges.
KEYWORDS
Pulmonary tuberculosis, invasive aspergillosis, type II diabetes, immunocompromised
INTRODUCTION
Pulmonary tuberculosis (TB) is a serious infection with long-term sequelae. Patients usually present with constitutional symptoms such as fever, night sweats and weight loss in addition to cough and haemoptysis [1]. Lung damage secondary to TB infection increases the risk of fungal infection, particularly aspergillosis, which manifests as aspergilloma in cavitary lesions. Aspergillosis is sometimes missed as it can mimic TB infection. In addition, acute invasive aspergillosis co-infection with TB in an otherwise young and healthy patient is uncommon[2]. In this report, we discuss the case of a young, healthy adult who had pulmonary TB (PTB) concomitant with invasive aspergillosis in the absence of previous lung damage.
CASE DESCRIPTION
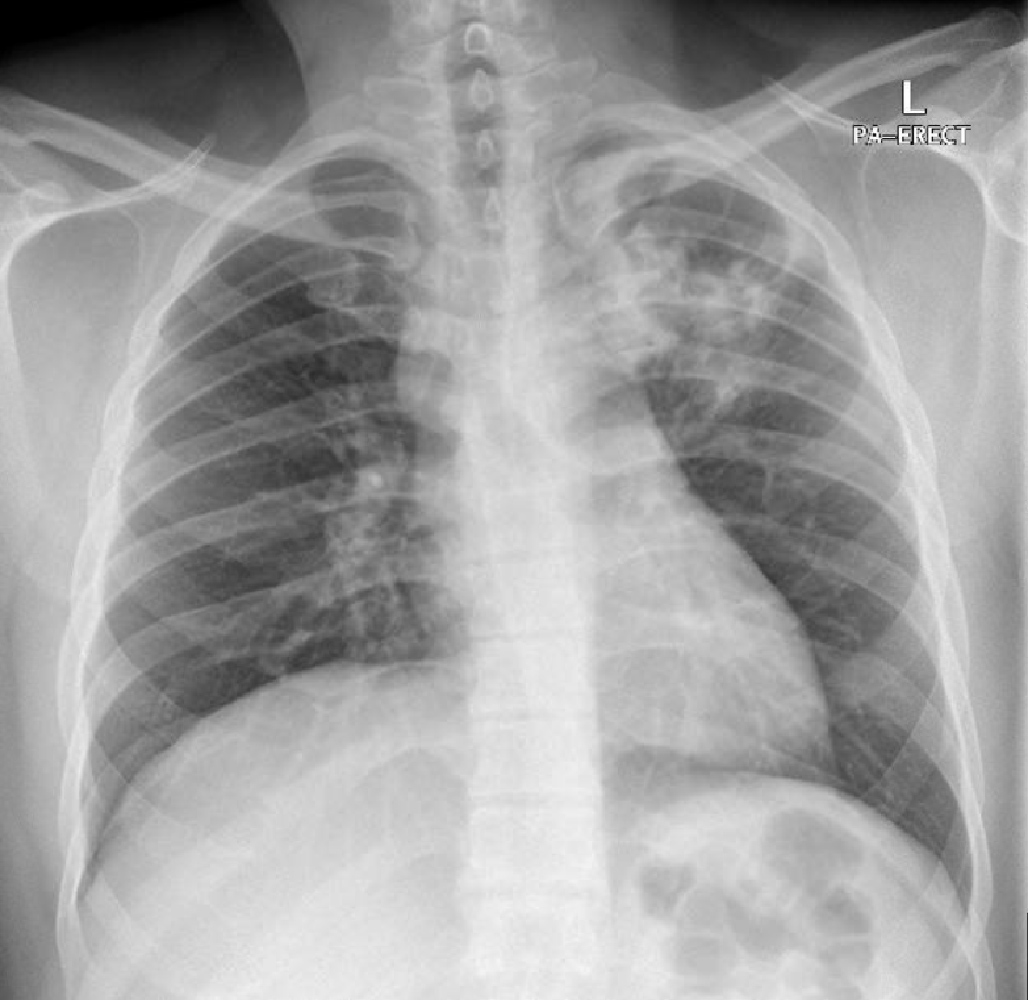
On physical examination, he had a fever of 38.3°C, respiratory rate of 20 breaths/min, heart rate of 99 beats/min, blood pressure of 125/83 mmHg, and oxygen saturation on room air of 98%. Chest examination was significant for bronchial breathing in the left upper zone. A chest x-ray revealed patchy consolidation involving the left upper lobe (Fig. 1). The laboratory findings were significant for leucocytosis of 14.9×103/µl (normal 4–10×103/µl) with neutrophil predominance, and a raised C-reactive protein of 197 mg/l (0–5). An HIV test was negative, HbA1c was 13.4 and the patient was diagnosed with type II diabetes. Given the upper lobe consolidation and the significant weight loss, the patient was screened for PTB with sputum samples taken for acid-fast bacillus (AFB) smear, culture and MTB (GeneXpert MTB/RIF). Inpatient bronchoscopy was arranged after the initial sputum TB work-up was negative, but again AFB smears and TB PCR were negative while the TB culture was pending. The patient responded initially to intravenous ceftriaxone and azithromycin and so was discharged to continue oral antibiotics and to follow-up in the outpatient clinic for the final sputum and bronchoalveolar lavage (BAL) TB cultures in 8 weeks.
Figure 1. Chest x-ray showing patchy consolidation involving the left upper lobe
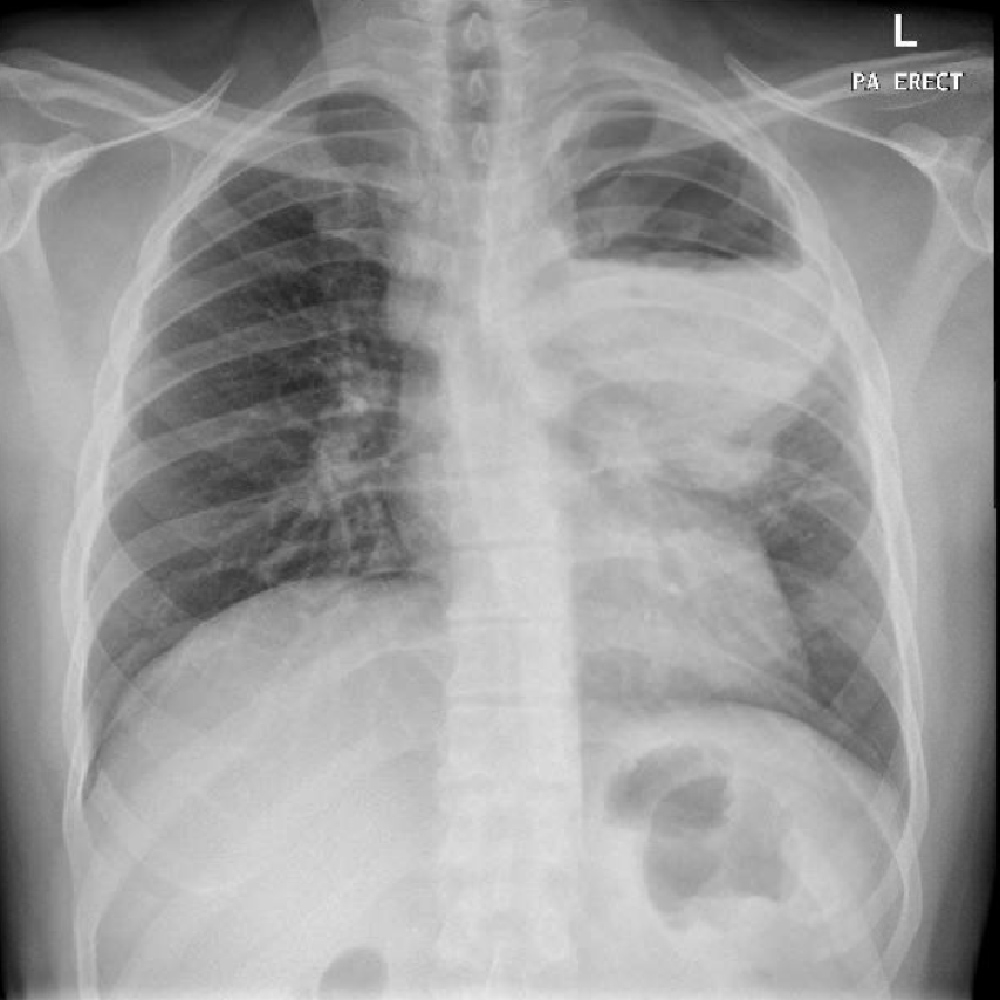
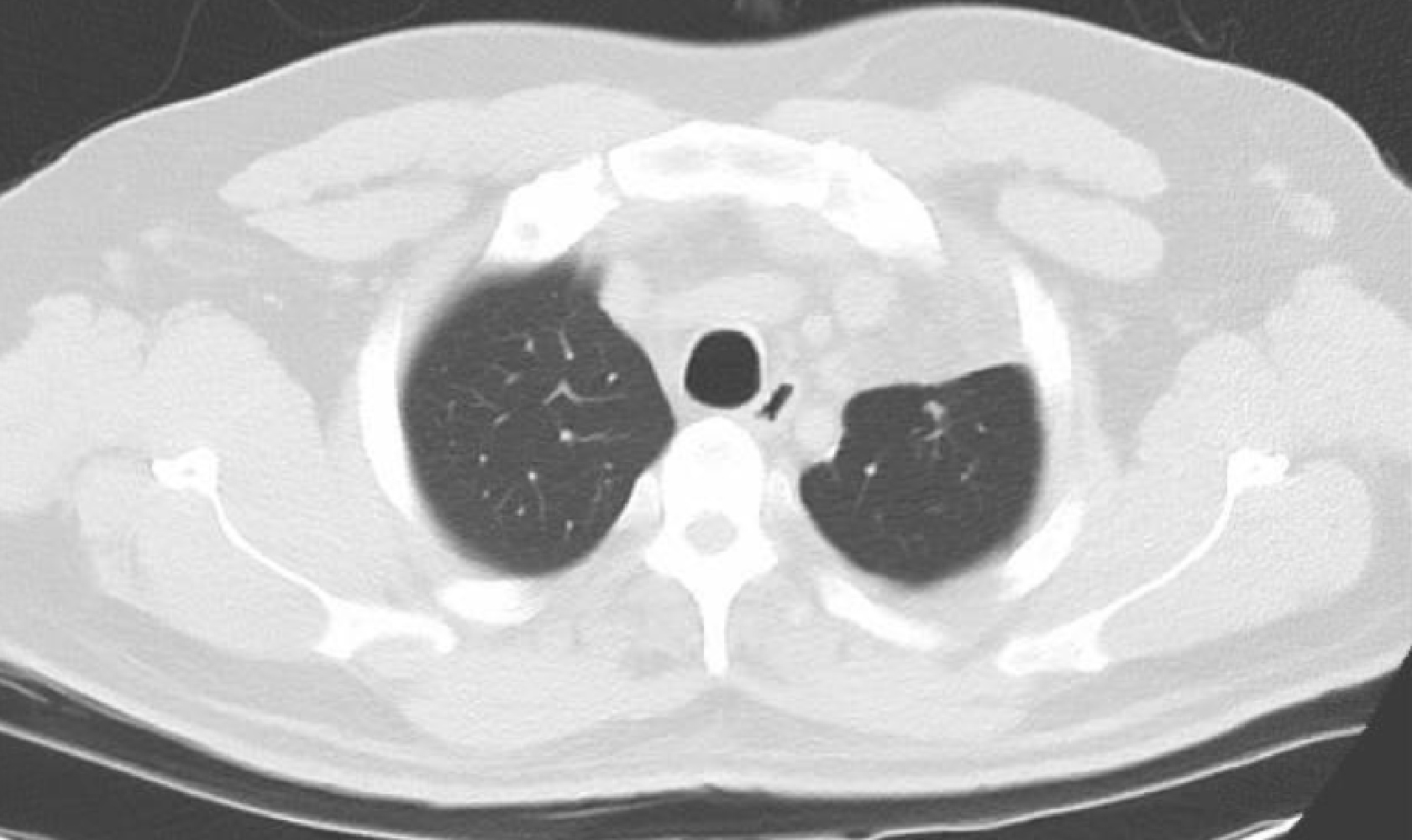
However, 2 weeks later, the patient returned to the ED with symptom relapse. The sputum was associated with blood streaks, he had mild chest discomfort and more SOB. He was febrile and tachycardic. Chest x-ray showed increasing left upper lobe pneumonic consolidation involving most of the upper lobe with the air–fluid level suggesting abscess formation (Fig. 2). Subsequently, computed tomography (CT) of the chest revealed evidence of necrotizing pneumonitis with an early lung abscess (Fig. 3).
Figure 2. Repeat chest x-ray showing increasing left upper lobe pneumonic consolidation involving most of the left upper lobe with an air–fluid level suggestive of an abscess
Figure 3. CT images of the chest showing complete collapse and consolidation of the left upper lobe with a large cavitating lesion with an air–fluid level showing peripheral enhancement
By this time, the previous BAL TB culture had been reported to be positive with a fully sensitive strain along with the growth of Aspergillus niger. The patient was started on first-line anti-TB medication (Rifafour) and the A. niger was considered to be contamination. Thereafter, lobectomy was planned given the large abscess size and persistent haemoptysis. Intraoperatively, adhesion between the parietal pleura and the lung was seen with a large abscess cavity containing pus. Later, A. niger was reported from the abscess, and a necrotizing granulomatous inflammation infiltrated by Aspergillus spp. was seen on histopathology. Because of the drug–drug interactions between voriconazole, rifampicin and isoniazid, the decision was made to commence liposomal amphotericin B for invasive pulmonary aspergillosis (IPA). Three days later, the patient’s creatinine had increased from 55 µmol/l (normal 62–106) to 110 µmol/l. Thus, liposomal amphotericin B was switched to voriconazole, and the anti-TB medications were adjusted to levofloxacin, isoniazid, pyrazinamide and ethambutol with monitoring of the voriconazole level and an electrocardiogram (ECG).
On a follow-up visit at 2 months, the anti-TB medications were stepped down to levofloxacin and isoniazid. The patient was gaining significant weight with no fever reported, improving radiologically, and maintaining good glycaemic control. He finished the 6-month course of voriconazole and subsequently levofloxacin was switched back to rifampicin. Four months later, CT of the chest was repeated and showed no evidence of an active infectious process (Fig. 4), and so anti-TB medications were stopped as the patient had improved clinically and radiologically.
Figure 4. CT of the chest after left lung upper lobe lobectomy, showing decreased left lung volume, shifting of the mediastinum to the left and no active infectious process
DISCUSSION
Tuberculosis (TB) is a well-known infectious disease caused by Mycobacterium tuberculosis aerosol deposition in the lung. It then progresses to active infection or becomes a latent infection that may later flare up if the host immune system is compromised [3]. PTB is the most common form of TB and patients commonly present with respiratory symptoms such as dry cough, or cough with sputum tinged with blood, and SOB. Patients with TB also develop systemic constitutional symptoms like fever, night sweats and weight loss [4]. Once PTB is suspected, the diagnosis is based on sputum microscopy for acid-fast bacilli, a molecular or nucleic acid amplification test (NAAT), and culture [5]. Furthermore, ATS/IDSA guidelines recommended flexible bronchoscopic sampling in adults with suspected PTB from whom a respiratory sample could not be obtained via induced sputum or which was negative. Our patient presented with symptoms, signs and radiological findings suggestive of PTB and therefore, extensive TB work-up was initiated. Subsequently, BAL TB samples confirmed the diagnosis [6].
PTB can present with a concomitant or superimposed infection, particularly bacterial infections. Attia et al. reported that 33% of patients with active PTB had a bacterial co-infection in an observational study involving 137 subjects [7], with Klebsiella and Pseudomonas spp. being the most common organisms. Other uncommon concomitant bacterial infections such as Salmonella typhi and Streptococcus milleri have been reported [8, 9]. Fungal co-infection with TB has also been described, with A. niger, A. fumigatus, Histoplasma capsulatum and Cryptococcus neoformans being the most common co-infections with PTB [10]. The risk of latent TB infection reactivation and the development of fungal infections may be increased in immunocompromised patients, such as those with malignancy and chronic diseases, and in those using excessive steroids and antibiotics [11].
Aspergillus, like other fungal infections, is not usually pathogenic but in immunocompromised patients and those with lung damage, can progress to active infection with devastating consequences. In PTB, particularly secondary TB, patients develop cavitary lesions which are vulnerable to Aspergillus infection [12, 13]. The small cavitary lesions allow the Aspergillus spores to colonize and grow, leading to the expansion or creation of new cavities, resulting in destruction [14]. Aspergillus infection is sometimes missed when a patient presenting with TB as the clinical features are similar (haemoptysis, weight loss, fever and night sweats). Additionally, the lack of clinical suspicion may result in misdiagnosis, and most importantly, the chronicity of the infection makes it indistinguishable from PTB [15]. Most Aspergillus cases are chronic, as it needs a damaged lung which typically occurs in PTB. Our literature search yielded only two patients with concurrent development of active PTB and IPA and both were significantly immunosuppressed [16]. It is noteworthy that concomitant isolation of M. tuberculosis and Aspergillus spp. from respiratory samples presents a complex dilemma and rigorous evaluation is required to avoid potential consequences [17]. However, co-infection remains rare, as Dellière et al. reported in their review [18].
The diagnosis of pulmonary aspergillosis requires microbiological and serological tests. The presence of a lung cavity with or without a fungal ball, in addition to the immunological response, might also warrant the diagnosis [19]. However, the diagnosis must be confirmed as treatment raises other problems. Anti-TB medications have many drug–drug interactions, and voriconazole and amphotericin interact with anti-TB drugs with severe adverse effects [20].
The diagnosis needs to be confirmed to avoid a missed diagnosis, delayed therapy or unnecessary administration of prolonged and potentially harmful antifungal medication. Voriconazole is considered the first-line therapy for the treatment of IPA. It is both a substrate and an inhibitor of CYP3A4, CYP2C9, CYP2B6 and CYP2C19 cytochromes, thus limiting its use due to a wide range of drug–drug interactions including with anti-TB medications, particularly rifampicin [21]. Therefore, we started our patient on liposomal amphotericin B to avoid this significant interaction but acute kidney injury developed and therapy was switched to voriconazole with close monitoring of levels. Rifampicin was reintroduced at the end of the 6-month course of voriconazole and the decision was made to treat PTB for 9 months given the extensive nature of lung involvement and the omission of rifampicin during the intensive phase of PTB treatment [22].
Interestingly, current evidence suggests an association between diabetes and the presence of Aspergillus spp. in the lung due to developing immunosuppression leading to persistence of infection and selection of resistant strains [23].
Our patient was newly diagnosed with type II diabetes with no apparent immunosuppression. In addition, the isolation of M. tuberculosis from BAL led initially to dismissal of the concomitant pathology. However, re-culture of Aspergillus spp. from the lung abscess and histopathological findings confirmed simultaneous IPA and PTB. The outcome was excellent with close follow-up and patient adherence to polypharmacy and appointments.
CONCLUSION
The possibility of concurrent active PTB and acute invasive aspergillosis in immunocompetent hosts should always be considered to avoid devastating consequences. Moreover, the co-administration of antifungal and anti-TB medications presents significant therapeutic challenges necessitating thorough evaluation and monitoring.