ABSTRACT
Spontaneous renal haemorrhage is a rare but severe condition known as Wunderlich syndrome (WS). The classic presentation includes sudden-onset flank pain, a palpable flank mass and hypovolaemic shock (Lenk’s triad). WS can be due to neoplasms, vascular diseases, cystic rupture, coagulopathies and infections. A contrast-enhanced CT scan of the abdomen is mandatory for diagnosis. Surgery is reserved for haemodynamically unstable patients and those with neoplastic disease. We describe a case of WS in an anticoagulated patient with chronic atrial fibrillation, diabetes mellitus type 2 and hypertension, who developed acute renal failure and severe anaemia, that completely resolved with conservative treatment and discontinuation of anticoagulation therapy.
LEARNING POINTS
- Wunderlich syndrome refers to spontaneous renal or perinephric haemorrhage.
- Contrast-enhanced CT of the abdomen is the gold standard for diagnosis.
- Surgery should be reserved for haemodynamically unstable patients or those with neoplastic disease.
KEYWORDS
Spontaneous renal haemorrhage, renal haematoma, Wunderlich syndrome, anticoagulation, flank pain, contrast-enhanced computed tomography
INTRODUCTION
Spontaneous renal or perinephric bleeding occurring in the absence of trauma is known as Wunderlich syndrome (WS) [1]. WS is a rare but severe condition which may require urgent nephrectomy. The classic presentation is Lenk’s triad, which consists of sudden-onset flank pain, a palpable flank mass and hypovolaemic shock, although most patients complain of non-specific symptoms, such as nausea, vomiting, fever and haematuria. Few cases of WS in anticoagulated patients have been reported in the literature [2, 3]. We describe a case of WS in a patient under warfarin treatment for chronic atrial fibrillation, who developed severe anaemia and acute renal failure with full recovery after conservative treatment and the discontinuation of anticoagulation therapy.
CASE DESCRIPTION
An 84-year-old woman presented to our emergency department (ED) with a complaint of sudden-onset right-sided flank pain without previous effort or trauma. The pain was severe and constant with an associated vasovagal reaction. She referred vomiting with reduced daily water intake and a persistent wet cough treated with ibuprofen in the previous week. She denied fever, haematuria and symptoms of urinary tract infection. She had a history of diabetes mellitus type 2, chronic atrial fibrillation under anticoagulant therapy, systolic hypertension, dyslipidaemia, obesity, hypothyroidism, osteoarthritis with multiple joint involvement, asymptomatic kidney stones and depression. Her medications included metformin and insulin, warfarin, carvedilol, valsartan, atorvastatin, levothyroxine, lansoprazole and venlafaxine.
The day before she had been discharged from our ED with a diagnosis of hypoglycaemic coma in diabetes mellitus type 2 which completely resolved with the administration of i.v. glucose solution, and x-ray evidence of a small left basal pneumonia without respiratory failure, performed for a persistent wet cough without fever or chest pain. A RT-PCR nasopharyngeal swab for SARS-CoV-2 resulted negative. Blood tests showed a slight increase in serum creatinine (1.20 mg/dl), blood urea nitrogen (60 mg/dl) and C-reactive protein (CRP) (1.04 mg/dl). Blood counts, serum electrolytes and liver enzymes were all within normal limits. A brain CT scan excluded acute damage and showed diffuse cerebral atrophy.
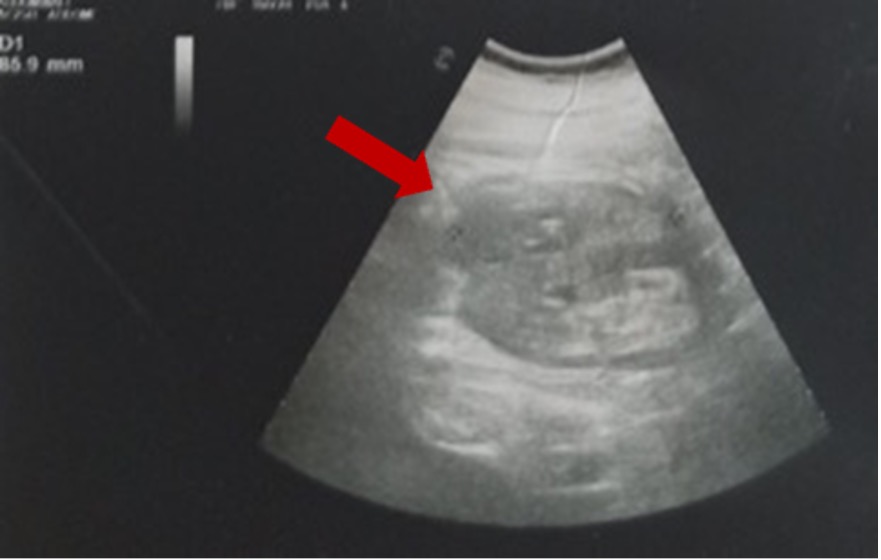
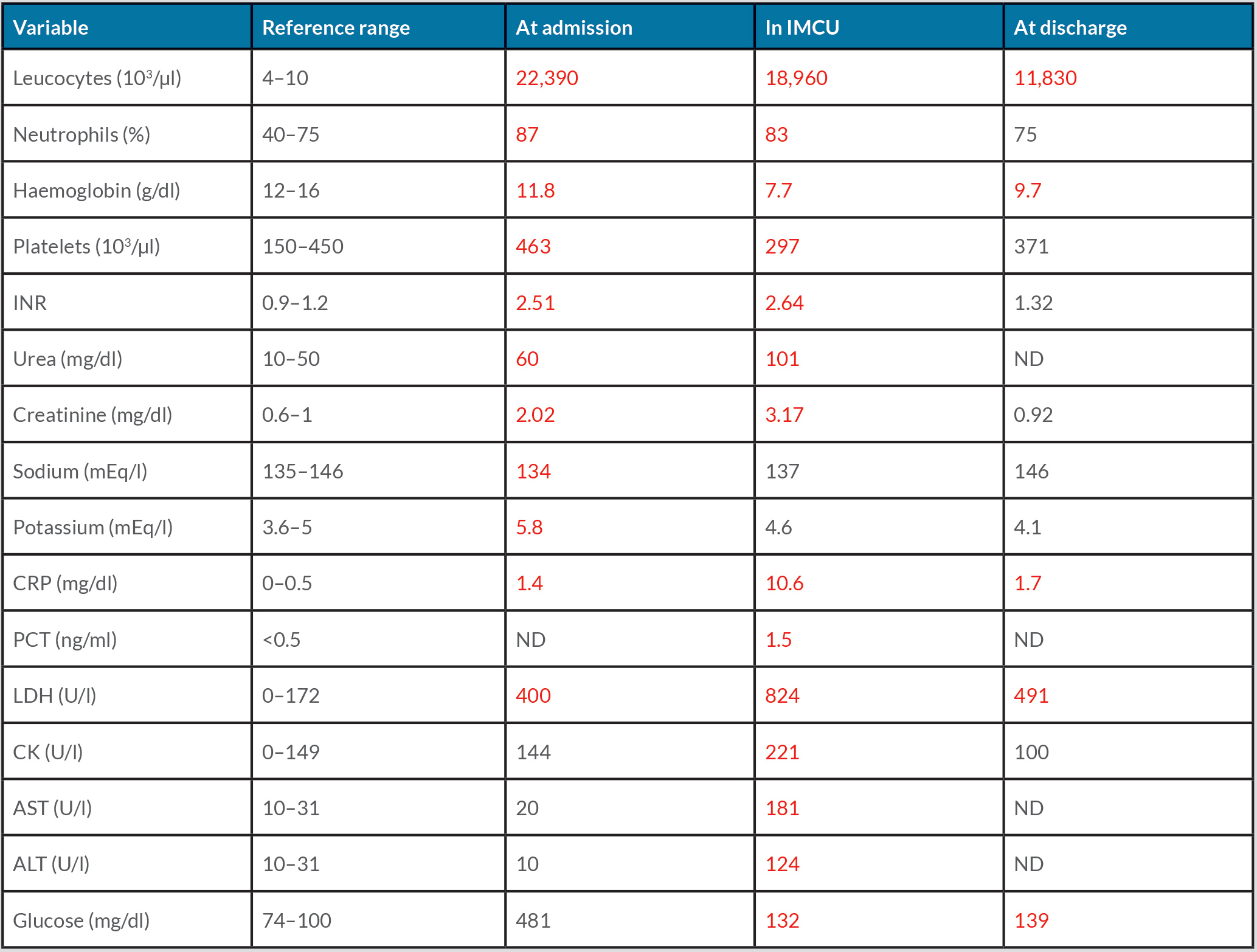
On admission, the patient was apyretic, displaying dry skin and mucous membranes. Blood pressure, heart rate, peripheral oxygen saturation and respiratory rate were 90/50 mmHg, 78 bpm irregular, 97% while breathing room air, and 16 breaths/min, respectively. Heart murmurs were absent, and chest examination was normal. On exploration, her abdomen was soft and depressible, but painful in the right flank with a positive right Giordano's sign. An electrocardiogram showed atrial fibrillation with medium ventricular frequency. Laboratory data revealed acute renal failure (creatinine 2.02 mg/dl) with hyperkalaemia (5.8 mEq/l), and hyperglycaemia (481 mg/dl) with metabolic lactic acidosis on venous blood gas analysis (pH 7.27, lactate 41 mg/dl, HCO3– 22 mmol/l). The blood count showed a slight normochromic normocytic anaemia (11.8 g/dl, MCV 89.3 fl, MCH 29.3 pg) with increased platelets, leukocytes and C-reactive protein (CRP), as reported in Table 1. The international normalized ratio (INR) was 2.51. Point-of-care ultrasound (PoCUS) documented a right kidney with an oedematous aspect and a coarse hypoechoic image in the superior pole in the absence of bilateral hydronephrosis and free abdominal fluid (Fig. 1).
Figure 1. Point-of-care ultrasound (PoCUS) showing an oedematous aspect with a coarse hypoechoic image in the superior pole of the right kidney (red arrow) in the absence of bilateral hydronephrosis and free abdominal fluid
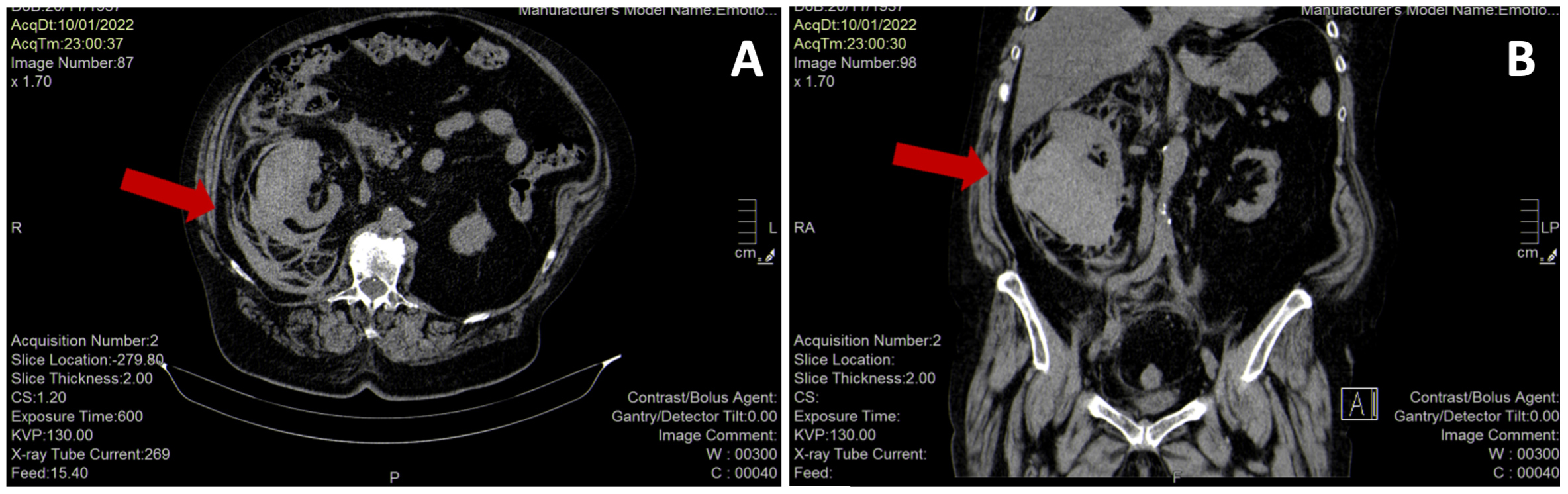
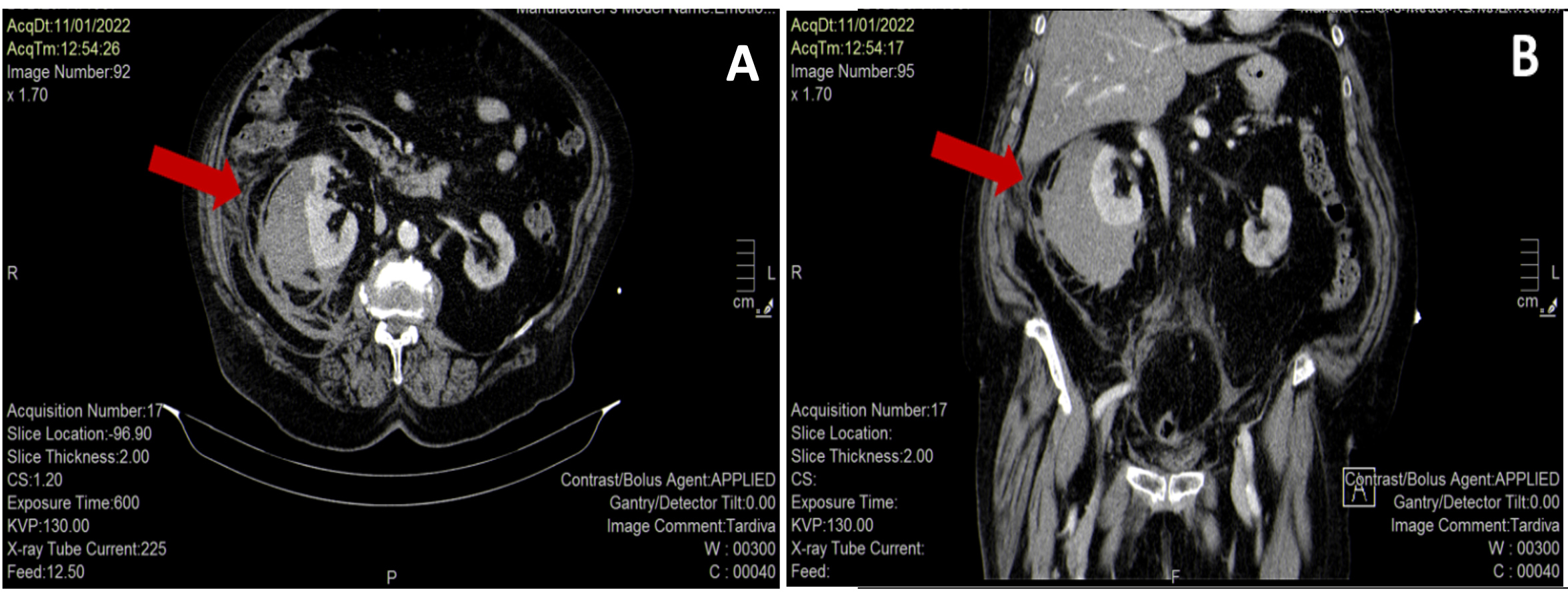
Abdominal CT without contrast confirmed a voluminous (11.5×9.5×12.5 cm) and patchy right kidney suspicious for acute renal haemorrhage (Fig. 2). The patient was immediately treated with aggressive volume resuscitation, broad-spectrum antibiotic therapy with ceftazidime 2 g i.v., and vitamin K 10 mg i.v., and was admitted to the intermediate care unit with the diagnosis of suspected WS. Laboratory data demonstrated a drop in haemoglobin to 7.7 g/dl and increased serum creatinine to 3.17 mg/dl with normal serum electrolytes, leucocytosis (22,400/µl) with a high CRP value (10.56 mg/dl) and procalcitonin (1.5 ng/ml). Liver enzymes, CK and LDH were elevated, as reported in Table 1. Repeat urine and blood cultures were negative. Urinary antigen testing for Pneumococcus and Legionella was negative. The diagnosis of WS was confirmed by contrast-enhanced CT of the abdomen, which showed a right perirenal subcapsular haematoma (9×9.5 cm on the axial plane and a cranial-caudal extension of 12 cm), without signs of active bleeding and without medium contrast elimination in the ultra-late phase (Fig. 3). There was no obvious renal mass or cystic disease. Surgery was excluded by urologists as active bleeding and neoplasms were absent, and the patient was haemodynamically stable after supportive therapy. A conservative strategy based on the discontinuation of anticoagulant therapy, the infusion of balanced crystalloids i.v., antibiotic treatment with ceftazidime adjusted for renal failure, and blood transfusion was planned. Support stocks were positioned since no antithrombotic prophylaxis could be administered. Daily echographic monitoring was performed, showing stability of imaging. Renal function gradually improved, and diuresis recovered. From day 3, haemoglobin remained stable at 8.5 g/dl without blood transfusion. On day 7, the patient was transferred to the internal medicine ward, and was discharged 7 days later in good clinical condition with a stable haemoglobin value (9.7 g/dl), normal renal function (creatinine 0.92 mg/dl) and well-controlled diabetes (Table 1).
Since there had been a major bleeding event, resumption of anticoagulation was not recommended, and the patient was discharged with enoxaparin at a prophylactic dose. Haemoglobin and platelet count at 7 days after discharge remained stable (10.6 g/dl and 370,000/mm3, respectively). Echographic follow-up at 3 months was planned.
Figure 2. Abdominal CT scan without contrast showing a voluminous (11.5×9.5×12.5 cm) and patchy right kidney (red arrows) suspicious for acute renal haemorrhage (panel A, axial view; panel B, coronal view)
Figure 3. Enhanced-contrast abdominal CT scan showing a right perirenal subcapsular haematoma (9×9.5 cm on the axial plane (A), and 12 cm of cranial-caudal extension (B)), without signs of active bleeding (red arrows)
Table 1. The patient’s laboratory findings at admission, during recovery in the Intermediate Care Unit (IMCU) and at discharge. Abnormal values are in red. ALT, alanine transaminase; AST, aspartate transaminase; CK, creatine kinase; INR, international normalized ratio; LDH, lactate dehydrogenase; ND, not determined.
DISCUSSION
The management of WS can be difficult, particularly in anticoagulated patients. First, clinicians should identify the aetiology of the spontaneous renal haematoma, and second, they need to define the management strategy. In the emergency room, PoCUS is useful for the evaluation of acute flank pain, but an emergent abdominal CT scan is mandatory if the patient presents haemodynamic instability with hypotension. Overall, the main cause of WS is neoplasms [4] (57–63%), most frequently angiomyolipomas (AML), followed by renal cell carcinomas (RCC). AML are benign renal tumours, clinically asymptomatic in most cases, although the larger ones (>4 cm) may have a high risk of bleeding. RCC are less common but are malignant [5]. In case of tumour, enhanced-contrast CT scan of the abdomen is the gold standard to identify the cause of haemorrhage and active bleeding [6]. Vascular diseases, infections, coagulopathies and cyst rupture must always be investigated and excluded [6]. Some cases are idiopathic but are probably due to the rupture of small cysts or a small extrarenal vessel, infection, inflammation or passed calculi. High risk pre-existing clinical conditions for WS are diabetes mellitus, hypertension, end-stage renal disease, a history of urinary tract infections, especially pyelonephritis, and renal cystic disease. The management of WS depends on the presence of active bleeding as well as the patient’s haemodynamic status. When active extravasation is detected on imaging, angiography and embolization are the preferred approach, with partial or radical nephrectomy reserved for neoplastic causes or refractory bleeding [7]. In all other cases, conservative treatment, based on early volume resuscitation with intravenous fluids and blood transfusion, immediate reversal of anticoagulation, pain control and antibiotics, associated with serial PoCUS and/or CT scanning is a reasonable option, as reported in our case [8].
In conclusion, clinicians should always consider WS in a patient with acute flank pain who develops unexplained haemodynamic instability and/or a drop in haemoglobin. If WS is suspected, a contrast-enhanced CT scan of the abdomen should be undertaken without delay and as reported in our case, conservative management with follow-up imaging can be a good strategy to avoid unnecessary surgery.