ABSTRACT
A 57-year-old man presented to the outpatient clinic with tremor, palpitations, weight loss and fatigue 1 week after receiving the first dose of the Oxford-AstraZeneca SARS-CoV-2 vaccine (ChAdOx1 nCoV-19). Laboratory studies showed a suppressed TSH with elevated total and free T4. Thyroid peroxidase and thyroglobulin antibodies were elevated but thyrotropin receptor autoantibodies were indeterminate. Thyroid scintigraphy with technetium Tc-99m pertechnetate revealed increased diffuse, symmetric uptake. The patient was treated with thiamazole 15 mg three times a day and propranolol with resolution of his symptoms and normalization of his thyroid function tests until discontinuation of the antithyroid drug 6 months after symptom onset.
LEARNING POINTS
- Thyroid autoimmunity triggered by SARS-CoV-2 vaccines is being increasingly recognized among patients with and without a history of autoimmune thyroid disease.
- Symptoms and signs of thyrotoxicosis, including fever and tachycardia, can be wrongly attributed to the systemic adverse events of these vaccines.
- Early recognition of this condition is mandatory to allow proper treatment with anti-thyroid medications and radioactive iodine when necessary.
KEYWORDS
Graves' disease, AstraZeneca, COVID-19
INTRODUCTION
It has been almost 2 years since the World Health Organization (WHO) declared coronavirus disease 2019 (COVID-19) a global pandemic, with over 490 million cases and 6.1 million deaths reported worldwide. Seven vaccines were developed, tested and approved in an unprecedented record time and in January 2021 immunization programs began at different rates in different countries. SARS-CoV-2 vaccines include the mRNA-based Pfizer-BioNTech Comirnaty and Moderna’s Spikevax; non-replicating viral vector (adenovirus) vaccines such as Oxford-AstraZeneca, Sputnik, Janssen and CanSino Convidecia; and the inactivated virus vaccine CoronaVac (Sinovac) [1].
Overall, these vaccines have significantly reduced the number of reported cases and mortality, but have also caused variable short- and long-term adverse events, including triggering underlying autoimmune conditions such as autoimmune thyroid disease.
To date, 20 cases of Graves’ disease (GD) occurring after SARS-Cov-2 vaccination have been reported, most of them following the administration of the Pfizer BioNTech mRNA vaccine. We herein report, to our knowledge, the third case of GD occurring after administration of the Oxford-AstraZeneca vaccine, which was successfully treated with thiamazole.
CASE DESCRIPTION
A previously healthy 57-year-old man presented to the outpatient clinic with a 5-day history of tremor, palpitations and a 3 kg weight loss as well as fatigue 1 week after receiving the Oxford-AstraZeneca SARS-CoV-2 vaccine. On physical examination, his blood pressure was 120/80 mmHg, his pulse was regular at 86 per minute, respiratory rate was 20 breaths per minute and his body temperature was 36º C. He appeared anxious but not in acute distress. Neck examination was remarkable for a palpable but painless, diffuse, symmetric, rubbery goitre approximately twice the size of a normal gland, with neither thrill nor murmurs, and no lymphadenopathies. Cardiopulmonary examination was normal, without murmurs or friction rubs. The abdomen was supple, with no hepatosplenomegaly and a somewhat increased peristalsis. Neurological examination was remarkable for a fine distal tremor and hyperreflexia.
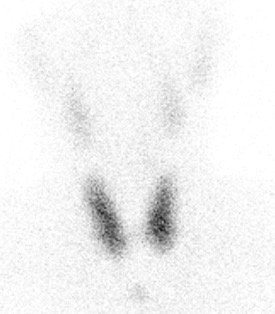
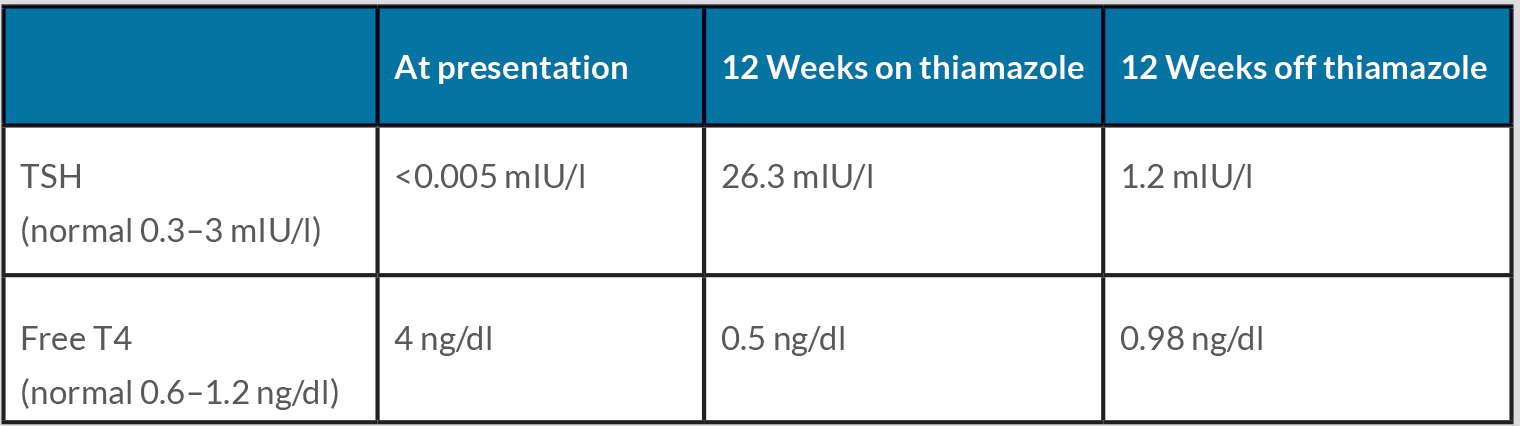
Laboratory work-up showed a suppressed TSH with elevated free and total thyroid hormones with positive anti-thyroglobulin (Tg) and anti-thyroid peroxidase (TPO) autoantibodies, and indeterminate thyrotropin receptor autoantibodies (TRAb) (Table 1). Blood chemistry and a complete blood count were within normal limits. Thyroid ultrasound showed a diffusely enlarged gland with increased vascularity. Thyroid scintigraphy with technetium Tc-99m pertechnetate revealed a diffuse goitre with increased uptake (Fig. 1).
Figure 1. Thyroid scintigraphy with technetium Tc-99m pertechnetate showing diffuse increased uptake
Table 1. Thyroid function tests at presentation and following 12 weeks on and 12 weeks after treatment with thiamazole
The patient was treated with thiamazole 15 mg three times a day and propranolol 20 mg twice a day. Symptoms of hyperthyroidism gradually resolved and after 3 months of treatment, his thyroid function tests showed frank hypothyroidism, and so the thiamazole was stopped. At his last follow-up visit, 3 months after discontinuation of thiamazole (6 months after initial presentation), he was found clinically and biochemically euthyroid.
DISCUSSION
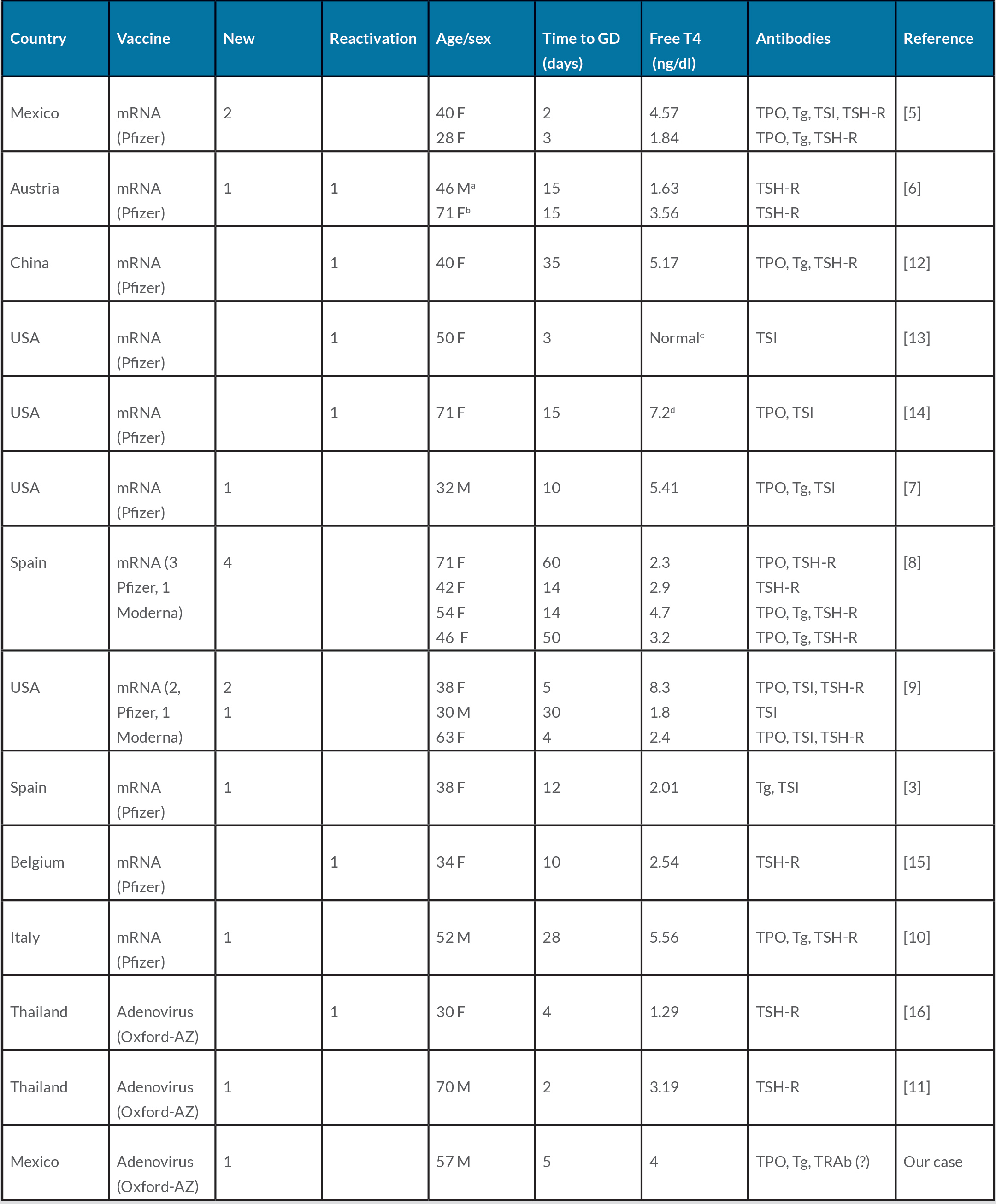
Since immunization against COVID-19 began in January 2021, 20 cases of GD occurring after vaccination have been reported (Table 2). Eighteen of these 20 patients (90%) had received an mRNA vaccine (Pfizer in 16 and Moderna in 2) and only two had been immunized with the Oxford-AstraZeneca non-replicating adenoviral vector vaccine (Table 2). Fourteen of these patients had no history of thyroid autoimmune disease (new onset, 70%), while 6 had been diagnosed with GD several years before vaccination and were all considered to be in remission (reactivation, 30%). Thus, our patient is the second reported case of new-onset GD occurring as a result of a non-replicating adenoviral vector vaccine.
SARS-CoV-2 infection is known to activate both the innate and adaptative immune response. Patients with a severe form of the disease usually develop an acute inflammatory state characterized by the synthesis and secretion of several proinflammatory cytokines that ultimately cause multiorgan failure. This so-called ‘cytokine storm’ is an exaggerated cellular immune response that more frequently than expected results in autoimmune conditions including rheumatoid arthritis, systemic lupus erythematosus, multiple sclerosis and haemophagocytic lymphohistiocytosis [2].
Several autoimmune adverse events have been reported in patients receiving both mRNA and adenoviral vector COVID-19 vaccinations, including immune thrombocytopenic purpura, autoimmune hepatitis, Guillain-Barre syndrome, IgA nephropathy, aplastic anaemia, autoimmune bullous disease, rheumatoid arthritis and systemic lupus erythematosus [3]. Our patient presented with the classic symptoms and signs of hyperthyroidism less than 1 week after receiving the Oxford-AstraZeneca COVID-19 vaccine, with clinical and laboratory features consistent with GD. Although SARS-CoV-2-induced subacute thyroiditis has also been reported [4], we are certain that our patient had GD, mainly because of his diffusely hypercaptating thyroid scan.
GD has been reported in 14 patients with no prior history of autoimmune thyroid disease [3, 5–11], while the remaining 6 had previously been diagnosed with hyperthyroidism but were in remission and were thus considered to have reactivations [6, 12–16]. The mean age of these patients was 47.3 ± 14.8 years (range 28–71), 15 were women and 5 were men. The median time from vaccination to the onset of symptoms was 16 days (range 2–60). Anti-thyroid peroxidase (TPO) and anti-thyroglobulin (Tg) autoantibodies were present in 11 (55%) and 8 (40%) patients, respectively, while positive thyroid stimulating immunoglobulin (TSI) or TSH-receptor antibodies were present in 100%. No significant clinical or biochemical differences between the new onset and the reactivation cases were identified.
Including the present case, there are three reports of GD occurring after immunization with the Oxford-AstraZeneca vaccine, which accounts for only 14.2% of the cases of SARS-CoV-2 vaccination-induced autoimmune thyroid disease. Although the sample size is not large enough to allow a formal statistical analysis, all three patients receiving this adenoviral vector vaccine developed symptoms of GD less than 1 week after immunization, in contrast to the 18 mRNA vaccine-induced cases, where time to onset was as long as 60 days, with a median of 18 days.
Of the two previously reported cases of AstraZeneca vaccine-induced GD [11, 16], one occurred in a female patient who had a prior history of autoimmune hyperthyroidism but was in remission and the other one in a male patient with no history of thyroid diseases. Both patients still required thiamazole to maintain euthyroidism 30–60 days after the onset of GD. In contrast, our patient responded rapidly and completely to thiamazole to the point of becoming frankly hypothyroid after 3 months of treatment. A few weeks after discontinuation of thiamazole, his thyroid function tests returned to normal and have remained so up to his last visit 8 months after the diagnosis of GD.
Regarding the pathophysiology of these adverse reactions, mechanisms such as molecular mimicry, the production of particular autoantibodies and an adjuvant-mediated effect have been proposed. In an in vitro study, Vojdani et al. found that antibodies against the SARS-CoV-2 spike protein cross-react with TPO. Furthermore, there is a non-negligible sequence homology between TPO and several SARS-CoV-2 proteins, including the spike protein [17]. Adjuvants are well known to trigger autoimmune diseases such as GD in genetically susceptible individuals [17]. Although the novel mRNA vaccines do not include a traditional adjuvant, the liposome that transports the genetic material can bind toll-like receptors (TLR) and can trigger an aberrant immune response [18].