ABSTRACT
Bradycardia, renal failure, atrioventricular (AV) nodal blockade, shock, and hyperkalemia (BRASH) syndrome is a relatively new clinical entity. It is often underrecognized, underdiagnosed, and confused with other causes of bradycardia. Treatment of BRASH syndrome differs from the standard bradycardia algorithm in advanced cardiac life support (ACLS), and the cornerstone management remains treating the hyperkalemia, improving renal function by treating the underlying cause, withholding AV nodal blocking agents, and considering dialysis in refractory cases, as any single factor could precipitate the vicious cycle. Here we describe two cases of BRASH syndrome with different clinical presentations that were treated with conservative management: one case in a 77-year-old Japanese woman and the other in an 86-year-old man.
LEARNING POINTS
- BRASH syndrome is an underrecognized clinical entity that healthcare providers need to be aware of. A medication review, particularly of cardiac medications, including AV nodal blocking agents, is critical for diagnosing BRASH syndrome.
- The management principles of BRASH syndrome are conservative management, addressing the precipitating event or medications and correcting electrolyte derangements.
- The prognosis of BRASH syndrome is excellent with timely recognition and management.
KEYWORDS
BRASH syndrome, bradycardia, renal failure, atrioventricular nodal blocker, shock, hyperkalemia
INTRODUCTION
BRASH syndrome is characterized by a clinical pentad of bradycardia, renal failure, atrioventricular (AV) nodal blockade, shock, and hyperkalemia[1]. It is a vicious cycle precipitated by renal failure, leading to hyperkalemia and accumulation of AV nodal blockers like beta-blockers (BB) or calcium channel blockers (CCB). Both hyperkalemia and AV nodal blockers cause bradycardia and hypoperfusion, which make renal failure worse. This syndrome is an emerging clinical entity that can lead to catastrophic events if left untreated. Here we present two cases of BRASH syndrome.
CASE DESCRIPTION
Case 1
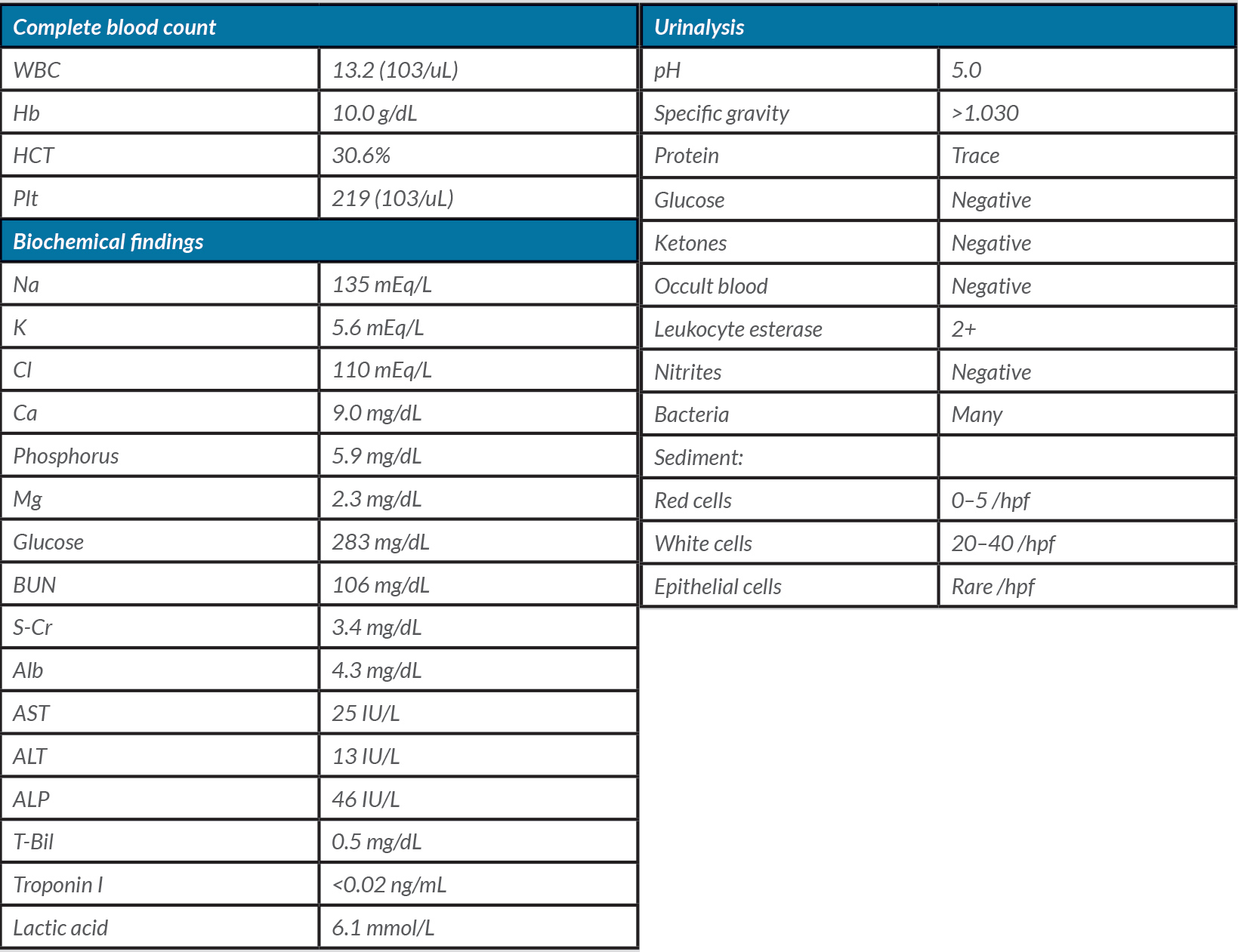
A 77-year-old Japanese female with type 2 diabetes mellitus, diastolic heart failure, permanent atrial fibrillation, and hypertension was admitted to our hospital after recurrent syncope. Each episode lasted less than a minute with full recovery of consciousness. Her medications included daily verapamil 240 mg, lisinopril 20 mg, and furosemide 20 mg. In the emergency department (ED), her vital signs were notable for hypotension, with a blood pressure of 90/40 mmHg and bradycardia of 30 beats per minute (bpm). Her cardiac rhythm was irregular with no murmur. The rest of her physical examination was otherwise unremarkable. Initial electrocardiogram (ECG) showed an idiopathic ventricular rhythm with a heart rate of 30 bpm (Fig. 1). Laboratory tests on admission are summarized in Table 1, which revealed mild leukocytosis, pyuria with bacteriuria, elevated blood urea nitrogen (BUN) (106 mg/dL), creatinine (3.4 mg/dL), and hyperkalemia (5.6 mEq/L). A diagnosis of BRASH syndrome was made. She received intravenous (IV) atropine 0.5 mg, regular insulin with dextrose and calcium gluconate, and IV fluids. All her medications were withheld and ceftriaxone commenced for a suspected urinary tract infection (UTI). Throughout her hospitalization, she had no recurrence of syncope and her renal function improved upon resolution of the UTI. The bradycardia gradually resolved and a pacemaker was therefore not required. She was restarted on low-dose verapamil with close cardiology outpatient follow-up.
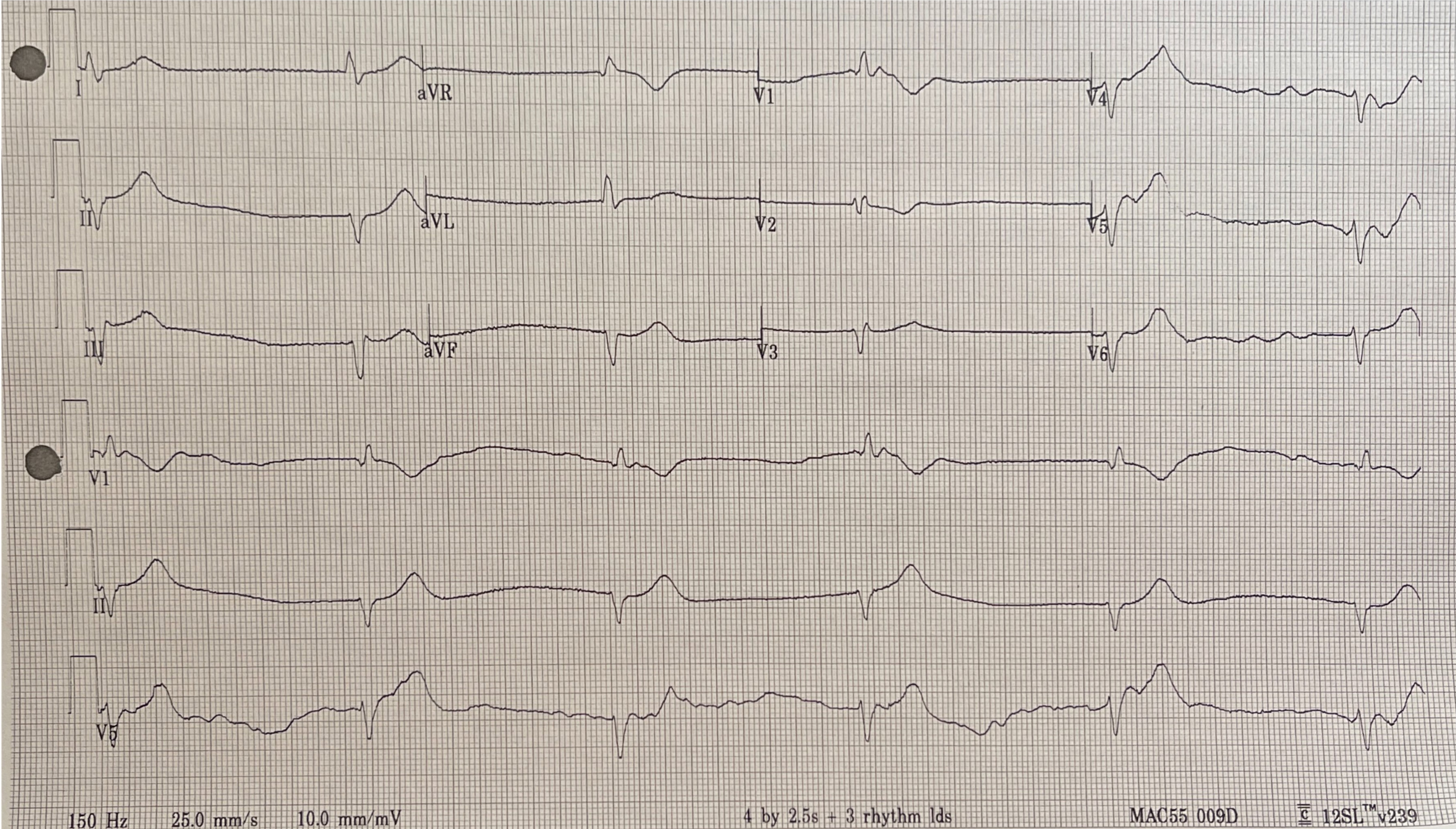
Figure 1. Electrocardiogram on admission, Case 1
Electrocardiogram on admission showing idiopathic ventricular rhythm with a heart rate of 30 bpm
Table 1. Laboratory values on admission, Case 1
Abbreviations: WBC: white blood cell; Hb: hemoglobin; HCT: hematocrit; Plt: platelet; Na: sodium; K: potassium; Cl: chloride; Ca: calcium; Mg: magnesium; BUN: blood urea nitrogen; S-Cr: serum creatinine; Alb: albumin; AST: aspartate aminotransferase; ALT: alanine aminotransferase; ALP: alkaline phosphatase; T-Bil: total bilirubin; hpf: high-power field
Case 2
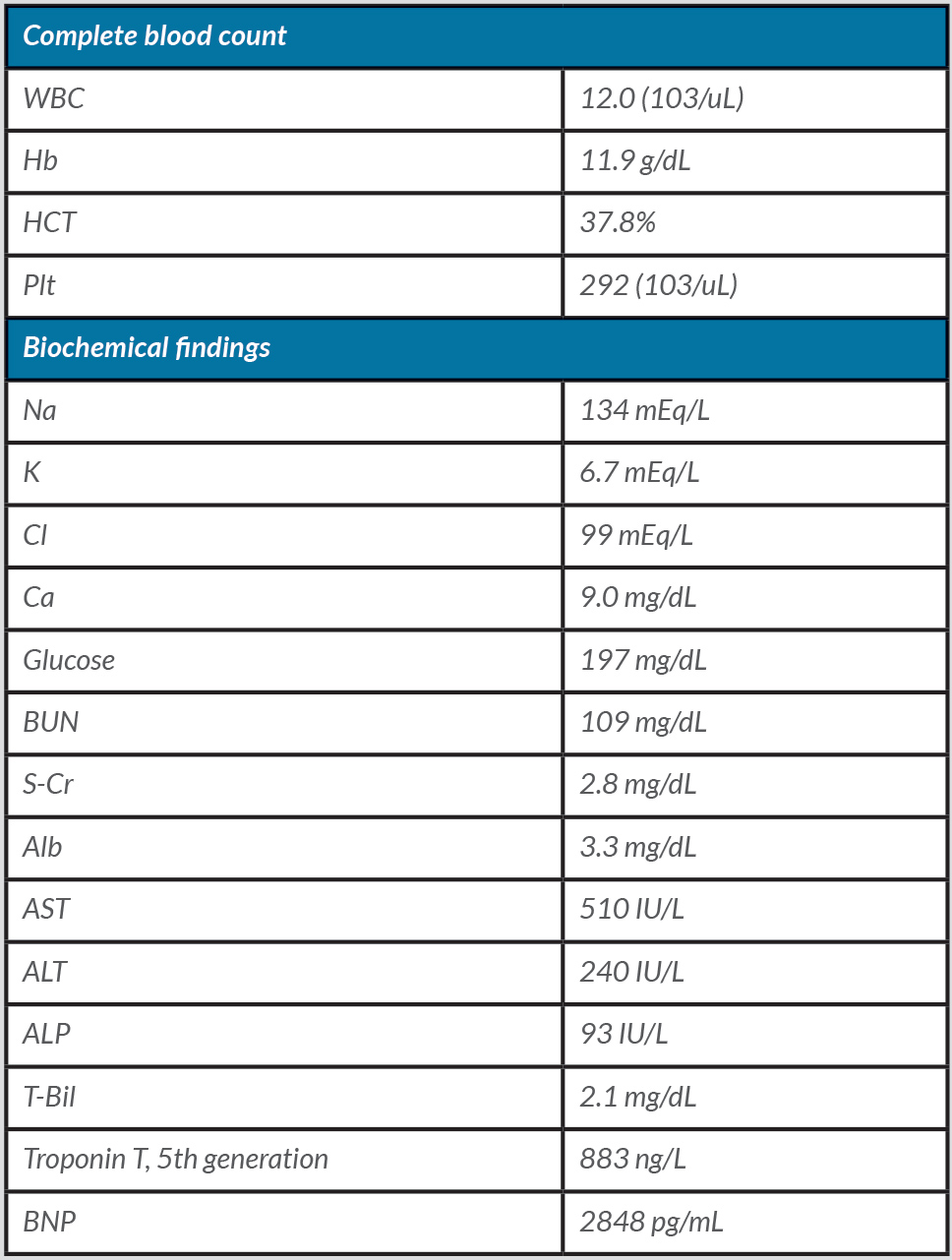
An 86-year-old Caucasian man with a history of heart failure with a reduced ejection fraction of 20–25% presented after an out-of-hospital cardiac arrest. He had recently been discharged from the hospital after balloon valvuloplasty with new medications prescribed, including metoprolol and lisinopril. On the day of admission, the patient felt palpitations, became pulseless, and had a cardiac arrest. Nursing home staff initiated advanced cardiac life support (ACLS) and return of spontaneous circulation was achieved after 2 minutes of cardiopulmonary resuscitation. In the ED, he had a junctional rhythm with a heart rate of 30–40 bpm (Fig. 2), hyperkalemia (6.7 mEq/L), elevated BUN (109 mg/dL) and creatinine (2.8 mg/dL). Pertinent laboratory values are summarized in Table 2. The patient was then diagnosed with BRASH syndrome. He was treated with IV calcium gluconate, insulin, furosemide, sodium zirconium cyclosilicate, and IV fluids, which improved his heart rate resulting in conversion to sinus rhythm. Metoprolol and lisinopril were withheld on admission. Following an electrophysiology consultation, the patient was not deemed a suitable candidate for a pacemaker, given his bradycardia had improved with correction of the metabolic derangements. Unfortunately, the patient subsequently died due to a hospital-acquired infection.
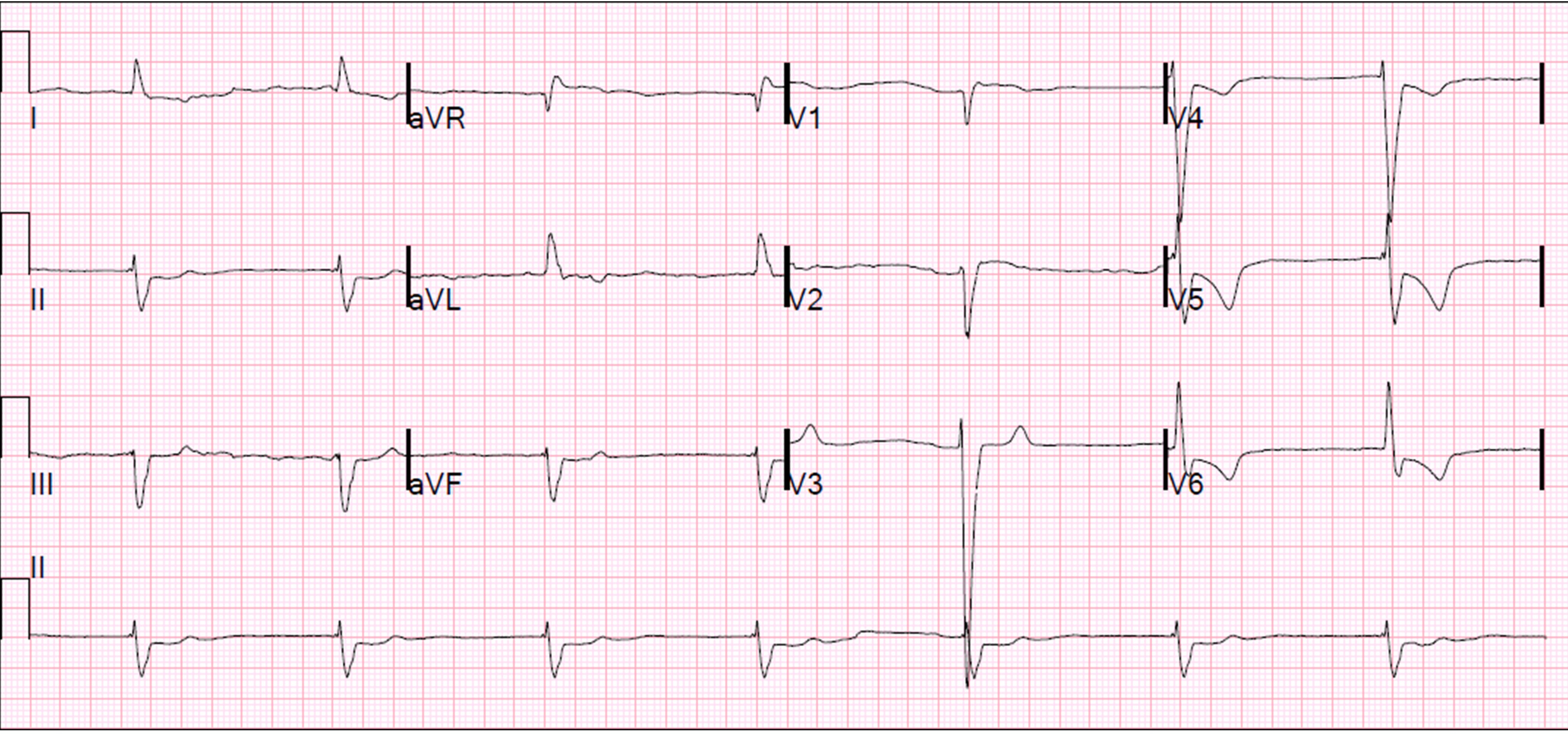
Figure 2. Electrocardiogram on admission, Case 2
Electrocardiogram on admission showing junctional rhythm with a heart rate of 30–40 bpm.
Table 2. Laboratory values on admission, Case 2
Abbreviations: WBC: white blood cell; Hb: hemoglobin; HCT: hematocrit; Plt: platelet; Na: sodium; K: potassium; Cl: chloride; Ca: calcium; BUN: blood urea nitrogen; S-Cr: serum creatinine; Alb: albumin; AST: aspartate aminotransferase; ALT: alanine aminotransferase; ALP: alkaline phosphatase; T-Bil: total bilirubin; BNP: brain natriuretic peptide
DISCUSSION
In this report, we summarized two cases of BRASH syndrome with different clinical presentations. Case 1 presented with complaints of syncope, whereas Case 2 had an out-of-hospital cardiac arrest. Clinicians should therefore keep the diagnosis of BRASH syndrome in their differential diagnosis when treating a patient with bradycardia.
The critical pathophysiologic characteristic of this syndrome involves a synergistic effect of hyperkalemia and AV nodal blockers resulting in bradycardia[2]. Due to the synergistic effect on heart rate, there have been case reports of BRASH syndrome with severe bradycardia at potassium levels around 5.0 mEq/L, which usually is not associated with significant arrhythmia[3]. Causative agents are typically BB or CCB, as in the present cases[4,5]. Although these classes of medications are generally well-tolerated and benign, they may cause significant AV nodal blockade when patients have precipitating events, such as systemic infection, leading to acute kidney injury, reduced clearance of drugs, and further declining renal function. Clinicians should be careful when starting patients on AV nodal blocking agents with a prior history of chronic kidney disease or with any concerns for acute kidney injury, as it could increase the risk of developing this syndrome.
Treatment of BRASH syndrome differs from the standard ACLS bradycardia algorithm, as atropine has no role in its management. The cornerstone of management is addressing all the individual factors that lead to this vicious cycle, including discontinuation of the offending medications, potassium shifting and elimination (e.g. administration of insulin/glucose, diuretics, potassium binders, sodium bicarbonate in acidosis), reversal of kidney injury, calcium gluconate for cardiac membrane stabilization, and vasopressors. For patients with profound bradycardia, inotropes such as epinephrine or isoproterenol may be used.
In conclusion, we presented two cases of BRASH syndrome with different clinical consequences. Patients with BRASH syndrome may present with nonspecific complaints, posing both diagnostic and therapeutic challenges. It warrants increased awareness among clinicians, as it may be treatable by early diagnosis and eliminating precipitating factors. While treatment of BRASH syndrome differs from the standard ACLS bradycardia algorithm, the cornerstone management remains to treat hyperkalemia, improve renal function by addressing underlying causes, and, most importantly, eliminate AV nodal blocking agents to stop the vicious cycle.