ABSTRACT
A patient with antisynthetase syndrome with pulmonary and muscular involvement was treated with immunosuppressive agents without corticosteroids. Rituximab was added to mycophenolate mofetil therapy with improvement in lung functional and imaging findings and normalization of creatine kinase levels.
LEARNING POINTS
- Antisynthetase syndrome associated with interstitial lung disease (ILD) and myopathy can be successfully treated without corticosteroids.
- A combination of several immunosuppressive agents can be a therapeutic alternative in case of refractory ILD.
KEYWORDS
Antisynthetase syndrome, interstitial lung disease, polymyositis/dermatomyositis, idiopathic inflammatory myopathy, mycophenolate mofetil, rituximab, corticosteroids.
INTRODUCTION
Antisynthetase syndrome (ASS) is defined as the presence of an antibody directed against one of several aminoacyl-transfer RNA (tRNA) synthetases. Clinical manifestations include inflammatory myopathy and extramuscular findings including interstitial lung disease (ILD), inflammatory arthritis and Raynaud phenomenon. ASS is usually treated initially with corticosteroids. A systematic review and meta-analysis of the treatment of idiopathic inflammatory myositis-associated ILD was carried out by Barba et al. in 2018 [1]. The authors advocated for the use of corticosteroids alone as first-line treatment for chronic ILD and corticosteroids in association with other immunosuppressive drugs for the management of rapidly progressive ILD. Prospective studies and randomized controlled trials are needed to precisely determine the efficacy of such drugs in the management of these severe diseases.
Here, we describe a patient who declined corticotherapy in case it caused glycaemic decompensation and other possible side effects in the setting of difficult-to-treat diabetes mellitus type 1 with continuous insulin infusion. The patient had never received any systemic corticosteroids.
CASE DESCRIPTION
Our patient was a 65-year-old woman with diabetes mellitus type 1 since 2010, who was receiving continuous insulin therapy for poor glycaemic control, with a high frequency of episodes of severe hypoglycaemia. The patient had also been previously diagnosed with ankle arthritis and erythema nodosum of the lower limbs (1995), scleritis (1996), primary Raynaud's phenomenon (normal periungual capillaroscopy in 2009), left-convex scoliosis of the spine, and upside-down stomach. She was the mother of two daughters, one of whom has systemic lupus erythematosus (SLE) and the other diabetes mellitus type 1, erythema nodosum and sarcoidosis. Since 2012, our patient had experienced respiratory symptoms with intermittent NYHA I–II exertional dyspnoea and cough with poor production of mucus. Respiratory function was characterized by restrictive lung disease with a total lung capacity (TLC) of 4.26 (80%), FEV1 of 2.38 (83%), FVC of 2.92 (88%) and CO diffusion slightly reduced at 65%. A CT scan of the chest showed ‘ground glass’ parenchymal infiltrates not visible on conventional radiography, with suspicion of stage I sarcoidosis. In 2017, the patient had contracted hepatitis E with a favourable spontaneous course.
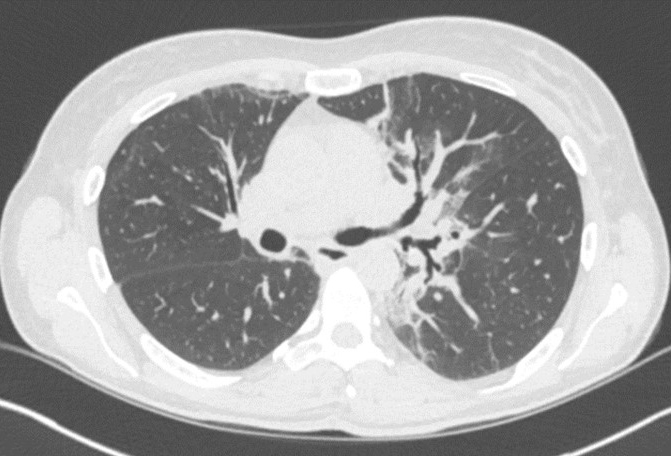
Progressive, slow worsening of dyspnoea was documented by spirometry demonstrating TLC of 3.72 (70%), FEV1 of 1.66 (61%), FVC of 2.10 (66%) and moderately reduced CO diffusion of 51% (June 2017). A CT scan revealed areas of bilateral parenchymal distortion with parahilar traction bronchiectasis (Fig. 1). Bronchoscopy performed the same year with bronchial biopsies and bronchoalveolar lavage (BAL) revealed a tracheobronchial tree within normal limits, ruling out a diagnosis of sarcoidosis.
Figure 1. CT scan (transverse section, March 2017) showing bronchiectasis with perihilar traction
In May 2017, tests were positive for the first time for anti-SSA autoantibodies (Ro-52) at 76 U/ml (normal <7–10 U/ml) and PL-7 at 16 U/ml (normal <6–10 U/ml), compatible with ASS. Laboratory tests showed creatine kinase (CK) elevated at 356 IU/l (reference value <160 IU/l), with sporadic myalgia.
A thoracic-abdominal CT scan (June, 2017) showed non-specific interstitial pneumonia (NSIP) with a predominantly inflammatory rather than fibrotic appearance with retractile aspects, compatible with an ASS type of pneumopathy. Further investigation including mammography, panendoscopy, and abdominal and pelvic ultrasound, excluded a paraneoplastic syndrome. We initiated immunosuppressive therapy with mycophenolate mofetil (MMF) in progressively increasing doses up to 1000 mg twice a day. The patient declined corticotherapy due to the risk of poor glycaemic control and possible side effects.
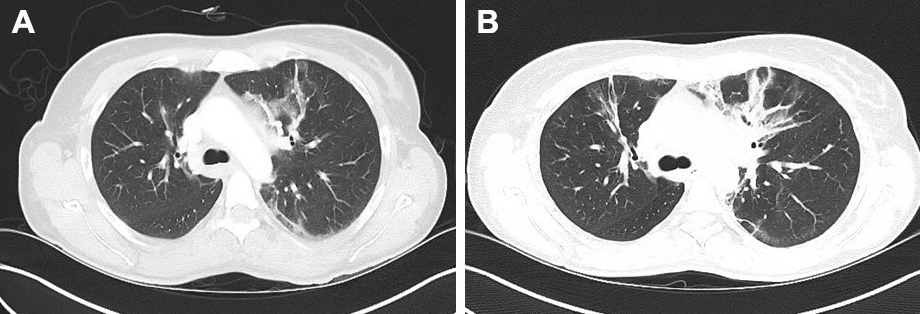
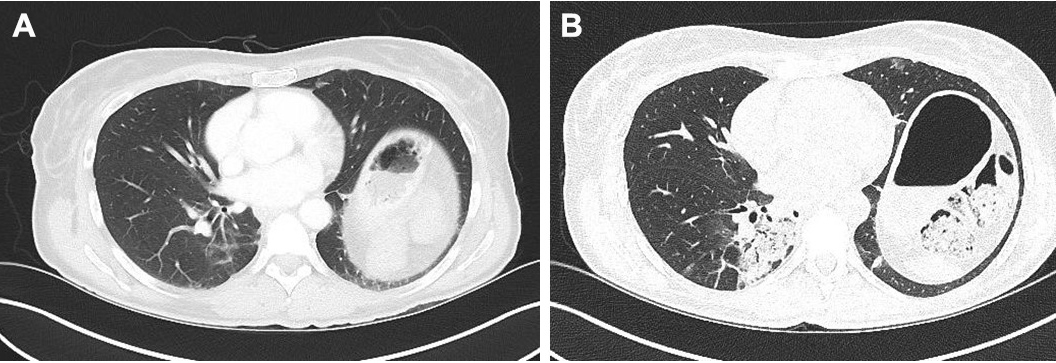
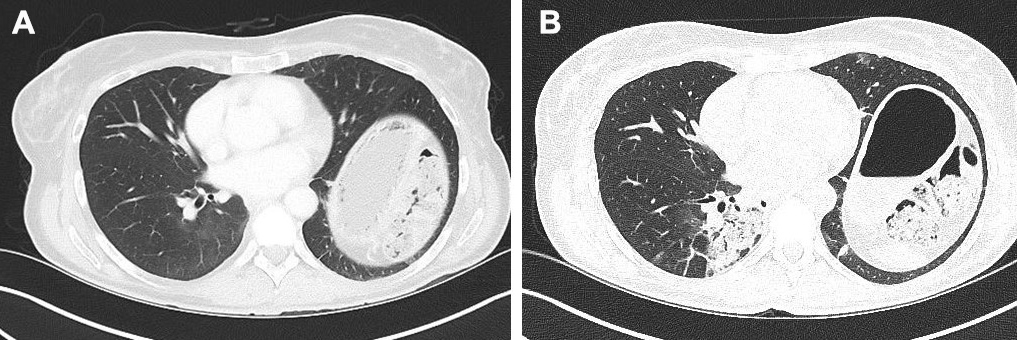
A high-resolution CT scan (December 2017) (Fig. 2; Fig. 3) showed increased ‘ground-glass’ parenchymal thickening in all lung fields with extension from the hilus to the subpleural regions and an air bronchogram.
Figure 2. Comparison of CT images (transverse sections) of the upper lung field taken in June 2017 (A) and December 2017 (B)
Figure 3. Comparison of CT images (transverse sections) of the lower lung field taken in June 2017 (A) and December 2017 (B)
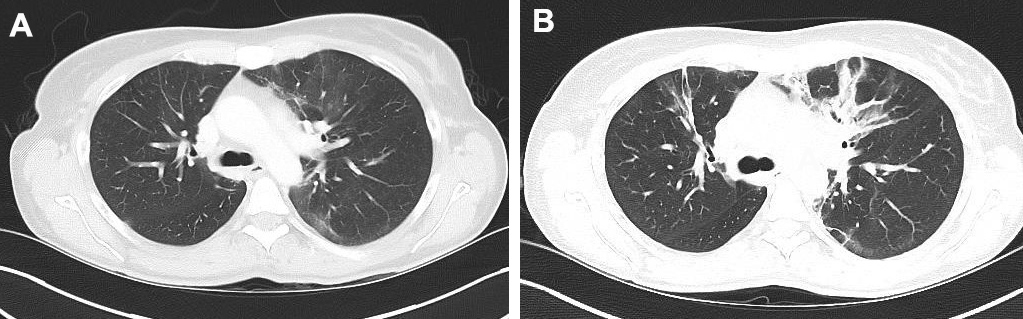
Therapy was potentiated by adding two i.v. infusions of 1 g rituximab (RTX) 2 weeks apart. A CT scan in July 2018 showed clear regression, with nearly complete disappearance of ‘ground glass’ infiltrates, reticulations and condensations in the different lung areas (Fig. 4; Fig. 5). Pulmonary function tests (PFTs) remained stable with TLC at 3.94 (74%), FEV1 at 1.85 (65%), FVC at 2.18 (60%) and CO diffusion moderately reduced to 58%. The patient, who is very athletic, noted a clear benefit regarding her physical abilities.
Ten months after the first doses of RTX, the patient developed marked myositis with elevation of CK up to 1,009 IU/l (normal <160 IU/l). Two further i.v. infusions of 1 g RTX were required, with rapid normalization of CK and remission of myalgia.
Figure 4. Comparison of CT images (transverse sections) of the upper lung field taken in July 2018 (A) and December 2017 (B)
Figure 5. Comparison of CT images (transverse sections) of the lower lung fields taken in July 2018 (A) and December 2017 (B)
DISCUSSION
The treatment of ASS with pulmonary and muscular involvement can be very challenging since there are no well-defined treatment schemes or precise therapy guidelines. Most data on the treatment of ASS are retrospective or uncontrolled.
Therapy is targeted to the systems and organs involved and based on the severity of symptoms [2, 3].
Controlled trials have not been performed to assess the superiority of corticosteroids compared with other immunosuppressive agents in the initial management of active disease. A 1993 study of 113 patients with idiopathic inflammatory myopathy (IIM) compared the efficacy of prednisone, methotrexate and azathioprine by clinical and autoantibody groups. Clinical response was mostly judged by myositis symptoms, not by lung disease symptoms. The first trial of prednisone alone for 4 weeks resulted in partial improvement in most patients with antisynthetase antibodies, but complete clinical response was rare [4].
The meta-analysis published by Barba et al. [1] reported that corticosteroids alone had >80% efficacy in the management of IIM-associated chronic-ILD (C-ILD). However, these apparently good results are likely biased, as common practice encourages the treatment of minor disease with corticosteroids alone and reserves immunosuppressive drugs for more severe or steroid-resistant ILD, or for patients expected to poorly tolerate corticosteroids due to comorbidities, especially diabetes or osteoporosis. Nevertheless, based on their results, the authors advocate the use of corticosteroids alone as first-line treatment[Q8] of C-ILD in patients without significant comorbidities. Second-line immunosuppressants, used in association with corticosteroids, were found to be moderately effective for the management of C-ILD, with a similar treatment effect (i.e., around 75% improvement) for cyclosporine A, azathioprine, RTX and tacrolimus.
Another retrospective study found that both azathioprine and mycophenolate were similarly effective in improving PFTs and reducing prednisone need in IIM-ILD, although azathioprine had slightly higher rates of adverse events and drug discontinuation than mycophenolate[5].
A number of small retrospective case series have been published, all of which report that in most patients RTX treatment results in disease stability or improvement in symptoms, imaging and/or PFTs [6, 7]. In some of these studies, the greatest improvement was seen in patients with a disease duration of less than 1 year or with acute exacerbation. ASS patients with a positive anti-Ro52 result may have a particularly robust response to RTX [7].
Marie et al. [8] in their retrospective study of seven patients with refractory ILD who had failed therapy with steroids or other immunosuppressive agents, observed an improvement after administration of RTX. The median daily dose of oral prednisone could be reduced in these seven ASS patients at 1-year follow-up, compared with the baseline of 20 mg/day.
In 2018, Doyle et al. [9] studied a cohort of 25 patients with ASS. In 18 patients, the diagnosis of ASS had followed the diagnosis of ILD. In 21 cases (84%), the principal indication for RTX use was recurrent or progressive ILD due to failure of other immunosuppressive agents. In four subjects (16%), RTX was chosen as the first glucocorticoid-sparing agent. At the time of starting RTX, 23 subjects were on prednisone (92%) and 12 (48%) were on additional concurrent immunosuppressive agents. In most patients, stability or improvement in CT imaging and/or PFTs (FVC%, TLC% and DLCO%) was observed at 1 and 3 years of follow-up. Furthermore, there was a significant steroid-sparing effect and RTX was generally well tolerated.
To our knowledge, this is the first report of an ASS patient with severe pulmonary and muscular manifestations who has never been treated with corticosteroids for the reasons described above.
Therefore, dual therapy with MMF/RTX can be a valid therapeutic option in such situations, even in the absence of corticotherapy, which is usually considered the first choice, at least initially and possibly in association with other later immunosuppressive therapy.
In our case, we used a combination of immunosuppressive agents, with potentially fewer side effects (mainly infectious) than parenteral cyclophosphamide. The short- and long-term risks are infections and (particularly for RTX ) decreased vaccine efficacy. The main advantage of this effective treatment (with no data available for comparison with steroid monotherapy) is that it avoids the typical side effects of corticosteroids.
A clinical trial of this approach could be interesting and useful considering the efficacy and generally good tolerance (few adverse effects) of the association of MMF with RTX.