ABSTRACT
Patients with severe iron deficiency, malabsorption or intolerance to oral iron are frequently treated with intravenous iron replacement. We report the case of a 42-year-old woman with non-erosive oligoarticular arthritis with antiparietal cell antibodies and iron deficiency anemia secondary to menorrhagia and unresponsive to oral iron preparations. She was treated with an intravenous infusion of ferric gluconate. After the first infusion of 125 mg (in 250 mL saline), she developed transient pain in her knee and wrist joints. When the dose was subsequently halved, the patient showed no adverse symptoms in the next four infusions and had normalized hemoglobin levels and iron indices. However, after a subsequent 125 mg ferric gluconate infusion she developed severe leg pain, muscular and joint stiffness, and functional impairment of her hands, right foot, and ankle. Laboratory tests showed a progressive increase in creatine kinase, transaminase, and C-reactive protein that normalized several days after the infusion. Although rhabdomyolysis is not reported among endovenous iron-induced adverse events, our findings suggest that intravenous iron infusions might have increased free iron generation promoting oxidative joint and muscular injury, which would explain the joint pain and stiffness, and rhabdomyolysis. Greater attention should be paid to the more frequent cases of myalgia occurring after iron infusion, which may underlie a rhabdomyolytic event requiring clinical observation.
LEARNING POINTS
- Rhabdomyolysis can occur not only after intramuscular injection of iron dextran but also after infusion of ferric gluconate.
- The generation of increased free iron following iron administration can promote oxidative joint and muscular injury.
- Leg cramps and myalgia, which are relatively common adverse events after iron infusions, might represent undiagnosed events of mild to moderate rhabdomyolysis requiring further investigation.
KEYWORDS
Ferric gluconate, rhabdomyolysis, non-transferrin-bound iron, adverse event
CASE DESCRIPTION
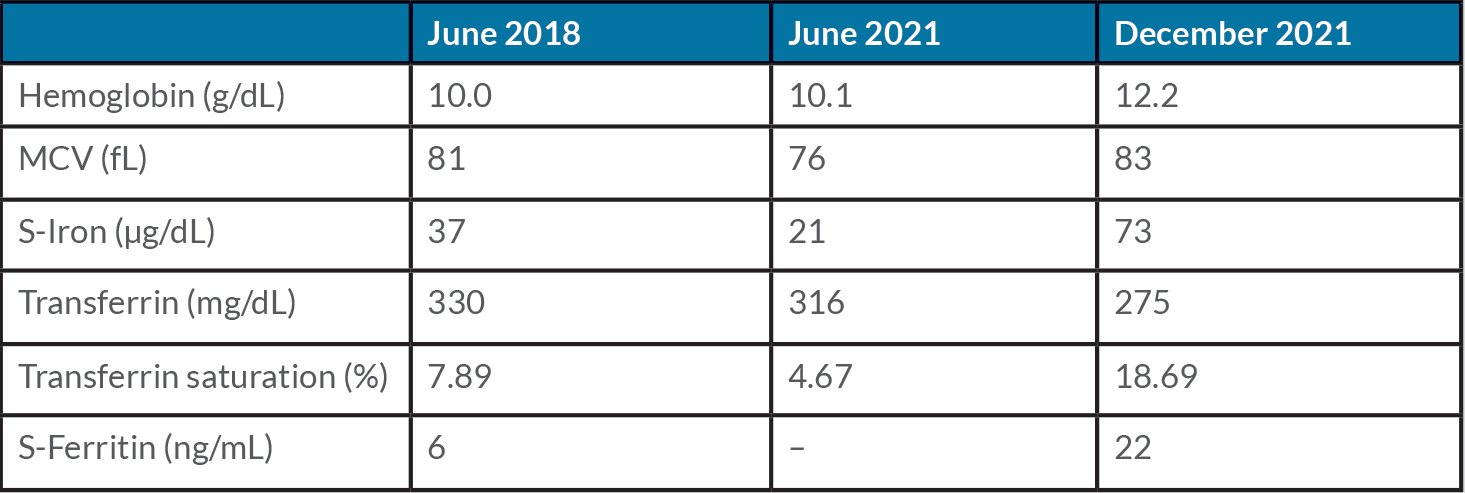
A 42-year-old woman was referred to our centre in 2019 for an iron deficiency anemia poorly responsive to oral iron, of many years’ duration and related to menorrhagia. Serum iron indices and blood count prior to 2019 are reported in Table 1. Since 2016, she complained of persistent arthralgia (hands, wrists, and knees) suggestive but not conclusive for rheumatoid arthritis (EULAR/ACR score=5). A diagnosis of non-erosive oligoarticular arthritis with positive antiparietal cell antibody was made. She attended the rheumatology unit and was regularly treated with hydroxychloroquine, and with steroids during pain exacerbation only. After ineffective attempts with various oral preparations, we prescribed an oral iron test in June 2021 (serum iron at baseline and 2 hours after 80 mg of oral ferrous sulphate administration), showing iron malabsorption (oral iron test: serum iron 21–47 µg/dL) that was likely related to autoimmune gastritis. A celiac test was negative. In July 2021, we started intravenous (IV) iron treatment. About 30 minutes after the first administration (125 mg of ferric gluconate diluted in 250 mL saline over 1 hour), the patient developed mild knee and wrist pain with spontaneous resolution within a few hours. We halted the infusions until October 2021, at which point we decided to resume the treatment using a half-dose (62.5 mg) every week to reach a total amount of 500 mg of iron supplementation to correct the anemia and iron deficiency status. No side-effects were reported. By the end of December, the blood count and serum iron indices had normalized (Table 1), indicating an adequate response to the iron infusions. A ferric gluconate infusion of 125 mg every month was then scheduled as maintenance treatment.
Table 1. Hemoglobin and iron indices before (June 2018 and 2021) and after intravenous iron treatment (December 2021)
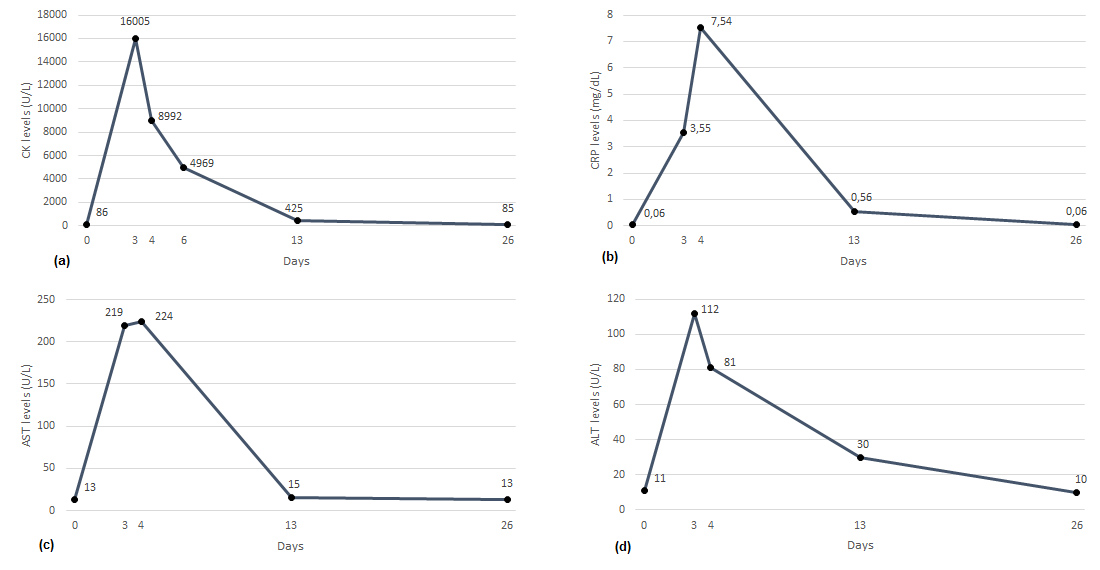
In January 2022, some 20 minutes after the end of the infusion she developed leg pain, joint and muscular stiffness, and functional impairment involving both hands. She described the leg pain as a painful hardening of the legs as if she had done intense and prolonged physical activity. Mild edema was present in the left hand, but she had no other clinical manifestations. After administration of 1 g of acetaminophen and 20 mg of methylprednisolone, she reported partial regression of hand symptoms but worsening pain in the right leg and foot causing severe functional impairment. At the time, biochemical parameters were normal, including blood count, creatine kinase (CK), C-reactive protein (CRP), liver function tests (Fig. 1), as well as renal function tests, sodium, potassium, and calcium. An IV infusion of ketoprofen was administered with partial benefit and she was discharged with oral ibuprofen and acetaminophen on prescription.
Figure 1. Time course at baseline and after ferric gluconate infusion of CK, CRP, AST, and ALT
However, after 3 days she presented to the emergency department of another hospital with persistent right leg pain and severe foot and ankle functional impairment. Laboratory findings showed increased CK, alanine transaminase (ALT), aspartate transaminase (AST) and CRP, hemoglobinuria, leukocyturia, and bacteriuria. Hemoglobin, mean corpuscular volume (MCV), platelets, fibrinogen, D-dimer creatinine, sodium, potassium, and calcium were normal, while leukocytes were slightly increased (12.2×109/L; neutrophils 10.00×109/L). A venous ultrasound ruled out deep vein thrombosis. She was treated with IV hydration and acetaminophen with partial regression of the symptoms. On the following day, CK, transaminases and CRP levels decreased and the leukocyte count and urine test normalized. She was discharged with vitamin B and acetyl-L-carnitine supplementation, adequate oral hydration, and acetaminophen as needed. The time course of the biochemical parameters is reported in Fig. 1, showing full normalization 26 days after the acute event. Phosphate and vitamin E levels were normal and the myositis autoantibody panel was negative. Electromyography showed the presence of leg myopathy and peroneal neuropathy fitting with compartment syndrome.
DISCUSSION
The patient’s clinical findings suggest that she suffered from rhabdomyolysis occurring after a parenteral ferric gluconate infusion. Leg cramps, myalgia, and arthralgia are reported among IV ferric gluconate side-effects, but rhabdomyolysis is not [1]. Similarly, iron formulations are not included in the list of drugs causing rhabdomyolysis [2].
In our patient, the CK timeline is aligned with what was expected in rhabdomyolysis, starting to rise within 12 hours after the onset of muscle damage, peaking in 1–3 days and declining 3–5 days after the cessation of muscle injury [2]. Also, the clinical course suggests a dose-dependent threshold for iron-dependent damage set at around 125 mg. To our knowledge, a single case of rhabdomyolysis occurring after intramuscular injection of iron dextran has been described in a patient with a history of malabsorption and vitamin E and selenium deficiency[3]. The authors suggested that intramuscular iron played a critical part in the development of muscle damage and that the associated deficiencies of vitamin E and selenium might have promoted iron-dependent oxidative damage to muscle membranes. IV iron infusions can lead to transient non-transferrin-bound iron (NTBI) generation that occurs through two pathways, rapid and delayed, consistent with direct and indirect (macrophage-mediated) iron release [4]. Iron–carbohydrate complex preparations with lower molecular weight (MW) that include both iron sucrose and ferric gluconate are less stable than higher MW complexes, resulting in a higher NTBI release after administration. A first peak represents predominantly the rapid release into plasma of weakly bound iron directly from circulating complexes (Pathway I). This can occur even when transferrin is not fully saturated and is dependent on the rate of release of iron from the formulation and the kinetic binding equilibrium of transferrin. The second peak occurs later when over 90% of low MW iron preparation has been taken up by macrophages, representing indirect ferroportin-mediated iron efflux from macrophages that fully saturates transferrin (Pathway II)[4]. Previous studies suggested that parenteral iron infusions mostly with iron dextran could exacerbate joint pain, synovitis, and arthralgia in patients with rheumatoid arthritis[5]. Accordingly, injection of iron dextran or sucrose led to significant exacerbation of joint inflammation along with iron deposition in the synovium of rats with adjuvant arthritis. We suggest that IV ferric gluconate infusions increased free iron generation that promoted oxidative joint and muscular injury, which explains the patient joint pain and stiffness and rhabdomyolysis. We also hypothesize that autoimmune disease increased the patient susceptibility to iron-induced oxidative stress[6]. We surmise that the relatively common muscular symptoms reported in patients receiving iron infusions might represent undiagnosed events of mild to moderate rhabdomyolysis requiring further investigation.