ABSTRACT
A 72-year-old woman, with anti-myeloperoxidase antineutrophil cytoplasmic antibody (ANCA)-associated vasculitis (AAV), presented with two episodes of spinal pachymeningitis (at two different levels 9 years apart, cervical in 2011 and dorso-lumbar in 2020) associated with aortitis and only demonstrated by F-18 fluorodeoxyglucose positron emission tomography/computed tomography (18F-FDG PET/CT). This association between aortitis and pachymeningitis in AAV appears exceptional. Moreover, the relapse of aortitis and pachymeningitis in 2020 was not accompanied by an increase in ANCA. This case demonstrates the value of 18F-FDG PET/CT in the management of AAV, providing evidence of the recurrence and distribution of lesions in various organs, including those with unexpected involvement.
LEARNING POINTS
- Involvement of large vessels such as the aorta is rarely associated with anti-myeloperoxidase antineutrophil cytoplasmic antibody (ANCA)-associated vasculitis (AAV), but has been described in a few cases. Possible aortic involvement should always be kept in mind while managing a patient with AAV.
- Pachymeningitis is rarely associated with AAV, but in case of unexplained and unspecific neurological symptoms in patients with AAV, such involvement should be considered.
- 18F-FDG PET/CT is a promising tool for the management of patients with AAV, allowing unexpected sites, undetected by usual examinations, to be highlighted. In contrast to giant-cell arteritis, this exam has not, until now, been included in the recommended/systematic work-up of AAV.
KEYWORDS
ANCA-associated vasculitis, 18F-FDG PET/CT, pachymeningitis, aortitis
CASE DESCRIPTION
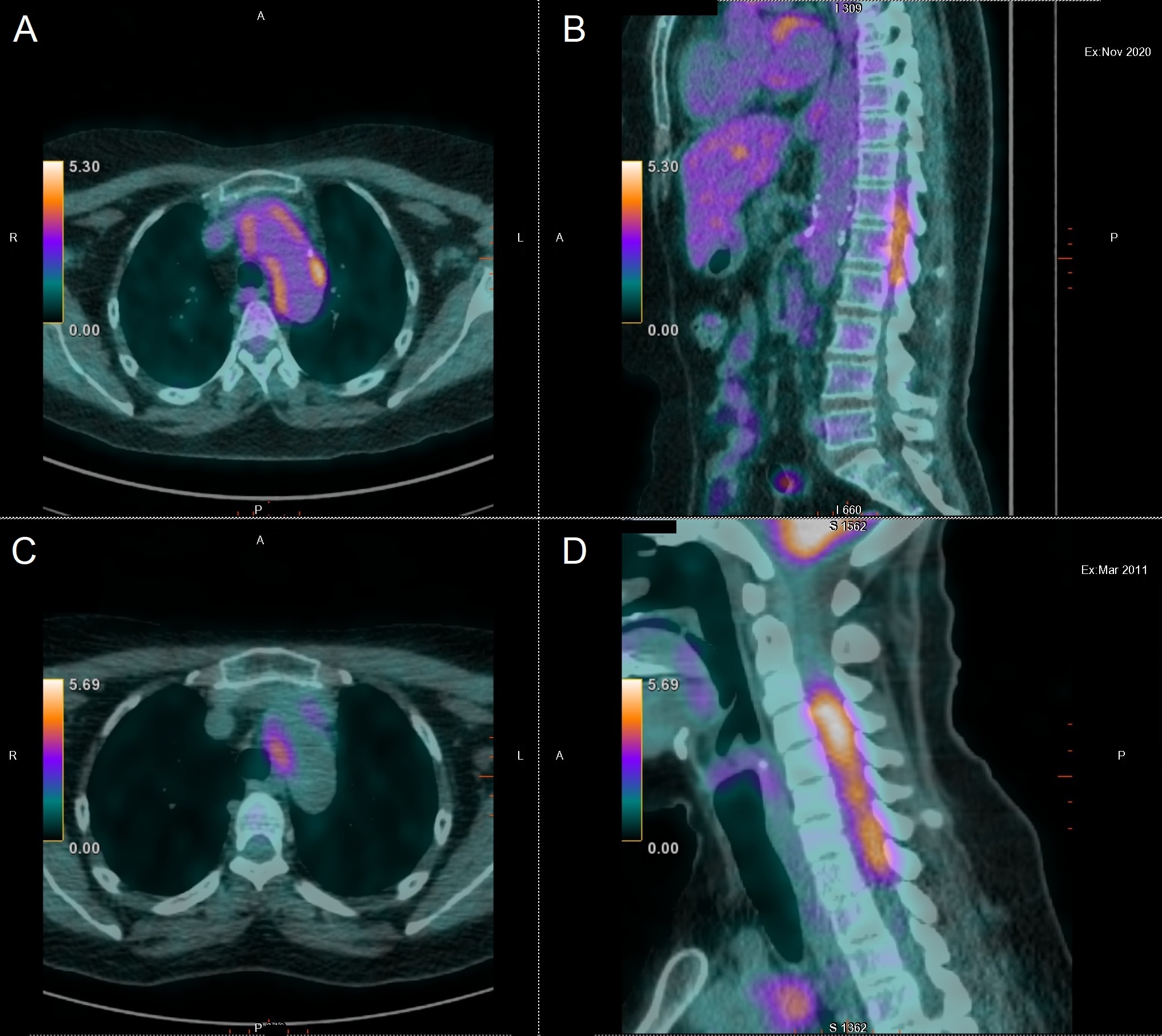
We present the case of a 72-year-old woman with a history of type 2 diabetes mellitus and dyslipidaemia who presented in 2010 with a prolonged inflammatory syndrome. Work-up revealed positive circulating anti-myeloperoxidase ANCA and 18F-FDG PET/CT demonstrated signs of inflammation localized in the thoracic aorta. Suspicion of giant-cell arteritis (GCA) justified a temporal artery biopsy, which demonstrated an arteriolar inflammatory cell infiltration without giant cells or granuloma. The patient was treated with corticosteroids and methotrexate. As previously described [1], aortitis and cervical pachymeningitis were discovered in 2011 on a 18F-FDG PET/CT performed for a refractory inflammatory syndrome in this patient (Fig. 1). At that time, the patient presented without any neurological symptoms and ANCA were negative. She experienced a long-lasting evolution of this pachymeningitis, for which she received successively methotrexate, cyclophosphamide and ultimately rituximab, which led to complete remission in 2015. In January 2020, 18F-FDG PET/CT performed for a new increase in the inflammatory parameters showed tracer uptake in the aortic wall, without any uptake in the spinal meninges. The inflammatory parameters decreased after treatment with corticosteroids, but recurred in October 2020, while ANCA remained negative. In November 2020, the patient started to complain of headaches, atypical vertigo and unsteadiness, and was hospitalized.
At admission, the patient had completely normal parameters. Clinical evaluation demonstrated that the vertigo was apparently of central origin with unsteadiness on walking, a slightly unstable Romberg test, and multidirectional nystagmus. The remainder of the clinical examination was unremarkable.
Figure 1. (A,B) 18F-FDG PET/CT performed in November 2020 showing aortic FDG uptake and marked hypermetabolism in the dorso-lumbar vertebral canal (D12 to L2). (C,D) 18F-FDG PET/CT performed in 2011 with high tracer uptake in the aortic wall and in the cervical vertebral canals
Methods and Procedures
In the emergency room, brain CT was unremarkable and blood tests only showed a mild increase in inflammatory parameters. During hospitalization, brain magnetic resonance imaging (MRI) was also normal.
18F-FDG PET/CT showed increased aortic FDG uptake and marked hypermetabolism in the dorso-lumbar vertebral canal (D12 to L2), with no uptake at the cervical level (Fig. 1). Recurrence of aortitis and pachymeningitis at a different level than in 2011 was diagnosed.
Spinal MRI was unremarkable. The cerebrospinal fluid examination demonstrated signs of mild inflammation without infection (proteins: 78 mg/dl; 12 leucocytes/mm3 and presence of oligoclonal bands).
The patient was treated with high doses of corticosteroids (intravenous methylprednisolone 500 mg over 3 days followed by oral methylprednisolone 40 mg daily) and methotrexate (15 mg weekly). Under treatment, the inflammatory parameters and the symptoms gradually improved. A new 18F-FDG PET/CT was carried out in March 2021 demonstrating clear improvement of the aortitis and disappearance of the dorso-lumbar pachymeningitis.
DISCUSSION
AAV is a systemic necrotizing small vessel vasculitis. Involvement of large vessels such as the aorta is uncommon in AAV, but has been described [2–5]. One should keep in mind that the well-known Chapel Hill classification of systemic vasculitides [6] relies on the size of the vessels predominantly – but not exclusively – involved, this concept supporting the fact that the involvement of large vessels can be encountered in a small-vessel vasculitis. The invasion of the aortic wall by inflammatory cells and granulomas resulting in granulomatous aortitis has been suggested as a possible mechanism of the disease [7]. Another possible explanation for the pathogenesis of aortitis is a necrotizing vasculitis involving the vasa vasorum of the aortic wall.
Inflammation of the meninges leading to pachymeningitis has been reported in association with granulomatosis with polyangiitis (GPA), a type of AAV generally linked to anti-proteinase-3 ANCA, but associated with anti-MPO in 10% of cases. In this setting, inflammation seems to affect the intracranial meninges more often than the spinal meninges [8,9]. The pathogenesis, although not well defined, may result from two distinct mechanisms. First, GPA commonly affects the sino-nasal structures and can cause granulomatous inflammation capable of invading neighbouring structures, such as the meninges. Second, it is a systemic disease, and vasculitis of small and medium arteries can occur in the central nervous system, including cerebral and spinal vessels, as anywhere else in the body [8].
A few cases of pachymeningitis specifically associated with AAV have been described in the literature [4,7,10]. Takenaka et al. reported the case of a 47-year-old Japanese woman who developed AAV complicated by a combination of aortitis and lumbar hypertrophic pachymeningitis, successfully treated with tocilizumab [10]. Parperis et al. described the case of a 71-year-old Hispanic woman who presented with aortitis and intracranial pachymeningitis due to AAV [7]. In a recent study by Delaval et al. reporting 50 cases of AAV revealed by a temporal artery biopsy, one of the patients presented with pachymeningitis [4]. Interestingly, AAV in our patient was also revealed by arteriolar involvement of the temporal artery; it may therefore also be classified as temporal arteritis revealing AAV [4]. In the retrospective case–control study involving 50 patients, Delaval et al. described the clinical, biological and histological presentations and outcomes of this specific subtype of vasculitis and compared these patients to controls with classic GCA. In patients with temporal arteritis revealing AAV, temporal artery biopsy showed fibrinoid necrosis or – as observed in our patient – small branch vasculitis in 23% of patients, but neither of these characteristics was evident in controls with GCA.
Only 18F-FDG PET/CT revealed the various manifestations in our case of AAV with aortitis and pachymeningitis previously affecting the cervical and later the dorso-lumbar meninges. No symptom directed attention to these localizations and spinal MRI was unremarkable at the time of the dorso-lumbar involvement. Therefore, 18F-FDG PET/CT in AAV appears to provide an extra benefit in addition to the usual work-up of AAV. This has already been demonstrated by Kemna et al. [11] who analysed 33 patients with AAV. In that study, 18F-FDG PET/CT in patients with active disease showed positive findings in multiple sites of the body, including some clinically unsuspected and difficult to assess otherwise [11]. In contrast to GCA in which aortic hypermetabolism is now considered both a clue to the diagnosis/a surrogate of temporal artery biopsy and a predictor of an increased risk of aortic complications at 5 years, the clinical relevance of aortic hypermetabolism and its impact on management has been, until now, unknown in the setting of AAV [12].
Finally, our patient's last relapse was not accompanied by an increase in ANCA, which is rather unusual.
In conclusion, the association of aortitis and pachymeningitis, although rare, can be a manifestation of AAV. This case demonstrates the value of 18F-FDG PET/CT in the management of AAV, providing evidence of the recurrence and the distribution of lesions in various sites, including some with unexpected involvement.