ABSTRACT
The left ventricular outflow tract is a region of the left ventricle that lies between the anterior leaflet of the mitral valve and the ventricular septum. Dynamic left ventricular outflow tract obstruction (LVOTO) has classically been observed in patients with hypertrophic obstructive cardiomyopathy (HOCM) where it occurs secondary to asymmetric septal hypertrophy and systolic anterior motion (SAM) of the mitral valve. However, there are some instances that lead to hypercontractility of the myocardium, and with a combination of other physiological conditions, result in SAM of a mitral valve leaflet, leading to LVOTO in the absence of phenotype.
We present such a case of an acute inferolateral wall myocardial infarction that was complicated by cardiogenic shock, requiring an intra-aortic balloon pump (IABP) and inotropic support which paradoxically provoked LVOTO. A reduction in IABP counterpulsation from 1:1 to 1:3 and the addition of intravenous fluids and a beta blocker resulted in significant improvement in blood pressure with rapid tapering of pressors.
Inotropes and IABP, although helpful in cardiogenic shock, have the potential to induce or worsen the LVOTO, which can lead to a vicious cycle of worsening hypotension and increasing adrenergic drive that further deteriorates myocardial viability. Timely diagnosis with an echocardiogram and the withdrawal of inotropic and IABP support has the potential to improve haemodynamics and, thereby, outcome.
LEARNING POINTS
- Dynamic left ventricular outflow tract obstruction (LVOTO) should be one of the differentials in patients with cardiogenic shock, especially if it is refractory in the setting of an intra-aortic balloon pump.
- The diagnosis of LVOTO by echocardiography should result in immediate implementation of therapy to eliminate the factors that can potentially intensify the obstruction.
KEYWORDS
IABP, LVOTO, HOCM physiology, SAM, cardiogenic shock
INTRODUCTION
Left ventricular outflow tract obstruction (LVOTO) has classically been observed in patients with hypertrophic obstructive cardiomyopathy where it occurs secondary to asymmetric septal hypertrophy and systolic anterior motion (SAM) of the mitral valve [1]. However, there are some instances that lead to hypercontractility of the myocardium, and with a combination of other physiological conditions, result in SAM of the mitral valve leaflet, leading to LVOTO in the absence of phenotype [2, 3]. We present such a case of an acute inferolateral wall ST-segment elevation myocardial infarction (STEMI) that was complicated by cardiogenic shock, requiring an intra-aortic balloon pump (IABP) and inotropic support which paradoxically provoked LVOTO.
CASE DESCRIPTION
A 79-year-old Hispanic woman with a past medical history of hypertension presented following an episode of severe substernal chest pain after which she collapsed and was resuscitated appropriately in the field. The initial rhythm in the field was asystole, followed by two shockable rhythms. In the emergency department, an ECG was performed which showed STEMI in the inferior leads with reciprocal changes in the anterior leads. Code STEMI was activated, and the patient was taken immediately to the cardiac catheterization laboratory. A left ventriculogram was performed which was significant for a left ventricular ejection fraction of 30–35% with severe hypokinesis of the mid-to-apical anterior wall, infero-apical wall, and apex in a pattern of Takotsubo cardiomyopathy. Cardiac catheterization revealed 100% occlusion of the left circumflex artery; a XIENCE drug-eluting stent was placed with restoration of Thrombolysis in Myocardial Infarction 3 flow.
Norepinephrine and dopamine infusions were begun as the patient was in cardiogenic shock with an elevated left ventricular end diastolic pressure of 32 mmHg. An IABP with one-to-one augmentation/counterpulsation was also initiated for a goal systolic pressure of 90 mmHg. Despite this, the patient’s extremities remained cold, with worsening hypotension.
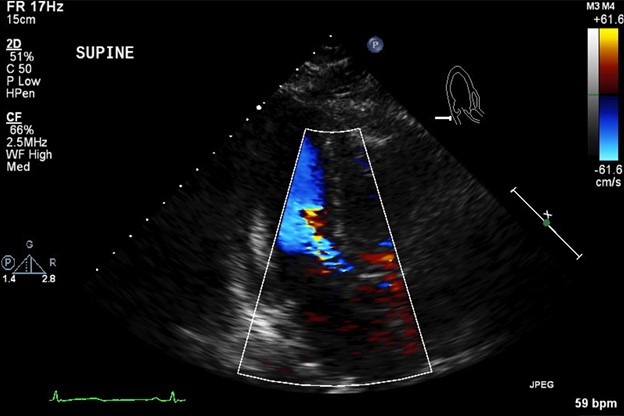
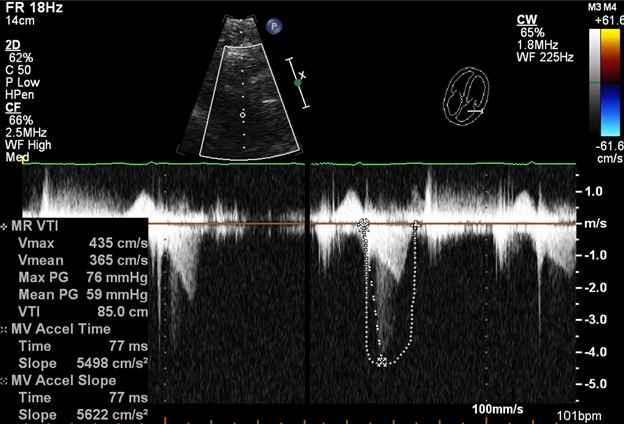
The following day, a transthoracic echocardiogram was performed which revealed a hyperdynamic anterior, anteroseptal wall and SAM of the anterior mitral valve leaflet with severe flow turbulence across the left ventricular outflow tract (Figs. 1 and 2). The patient was supine, hence imaging quality was limited. Clinical examination revealed a harsh 4/6 systolic murmur over the left lower sternal border. Double tapping of the carotid impulse was also noted. We switched IABP to 2:1 mode and the arterial wave form demonstrated a spike and dome appearance in the augmented beat.
Figure 1. Apical long axis view with colour flow showing severe turbulence at the left ventricular outflow tract
Figure 2. Transthoracic echocardiogram showing outflow tract obstruction with a spectral Doppler trace. The concave (dagger) shape of the waveform is seen on each systole. The continuous wave peak velocity reaches 4 m/s
At this time, the strategy was reversed. The patient was given intravenous fluids and the balloon pump was reduced to 1:3, which resulted in improvement in blood pressure with rapid tapering of pressors. In addition, a beta blocker was initiated, which resulted in further improvement of blood pressure, with improved urine output and warmth of extremities. The balloon pump was weaned off and removed over the next 24 hours. The patient remained haemodynamically stable off the pressors and was discharged to the nursing home on aspirin, ticagrelor, a high-intensity statin, an angiotensin-converting enzyme (ACE) inhibitor and a beta-blocker.
DISCUSSION
The left ventricular outflow tract is a region of the left ventricle that lies between the anterior leaflet of the mitral valve and the ventricular septum. Dynamic LVOTO is sensitive to changes in preload and afterload. Several cases have been reported in the literature showing an association between LVOTO and STEMI [4]. In inferolateral wall STEMI, the anteroseptal wall frequently becomes hypercontractile in compensation. In females, who generally have a small left ventricle, increased contractility of the anteroseptal wall can increase the flow acceleration in the left ventricular outflow tract. If these patients are subjected to physiological changes that increase contractility and dramatically reduce the afterload, it can produce LVOTO even in the absence of asymmetric septal hypertrophy. Moreover, it can cause clinically significant mitral valve regurgitation that decreases the afterload, further worsening the cardiac output.
IABP is one of the most widely used circulatory assist devices. It dramatically decreases afterload, and reduces cardiac work and myocardial oxygen demand while increasing diastolic coronary blood flow [5]. However, by doing so, IABP has the potential to compromise haemodynamics by inducing or worsening the LVOTO in the appropriate clinical scenario [6].
Our patient had an inferolateral wall STEMI with a hypercontractile anterior and anteroseptal wall. As she was hypotensive, multiple pressors were started and an IABP was placed in the cardiac catheterization laboratory. This paradoxically worsened the patient’s blood pressure by producing a physiology of obstructive cardiomyopathy. Recognizing the complete pathophysiology was paramount in improving haemodynamic stability.
The management of dynamic LVOTO includes increasing left ventricular volume with fluid administration, increasing afterload with a vasoconstrictor or by removal of an IABP [6], and decreasing heart rate and inotropy with beta blockers [7]. Inotropes are believed to cause or worsen LVOTO in 7–21% of patients, by promoting hypercontractility, accelerating blood in the left ventricular outflow tract, and worsening of SAM [8]. In our case, the patient’s haemodynamics were further improved with beta blockers and tapering of inotropes.
CONCLUSION
Post-inferolateral wall STEMI and compensatory hypercontractility of the anterior wall in the setting of IABP, which dramatically decreases afterload, can cause hypotension secondary to SAM and resultant hypertrophic obstructive cardiomyopathy physiology. If the condition is not recognized early, the use of vasopressors will further decrease blood pressure. Hence, it demands meticulous evaluation of the patient’s haemodynamics. In our patient, the dramatic bisfriens carotid pulse with a harsh left ventricular outflow systolic murmur guided us to further evaluate her cardiac physiology. The timeliness of the appropriate changes in the management resulted in stability of the patient.