ABSTRACT
Introduction: Severe haemophilia A is characterized by serious factor VIII deficiency (biological activity <1%) resulting in frequent spontaneous haemorrhage and abnormal bleeding after minor injury, surgery or tooth extraction.
Patient and Methods: We report the case of a 58-year-old patient with severe haemophilia A without inhibitors but with other comorbidities (HCV and HIV seroconversion), who underwent coronary angioplasty and stent implantation after acute myocardial infarction.
Results: Compared with previous therapy, Nuwiq® led to a reduction of about 20% in drug consumption (360,000 IU vs 540,000 IU per year) and in the annualized bleeding rate (ABR) (5 vs 15).
Discussion: Pharmacokinetic-guided personalized prophylaxis with Nuwiq® provided bleeding protection with good tolerability and a satisfactory pharmacokinetic profile in a patient with severe haemophilia A and comorbidities whose replacement therapy had to be adjusted because of other contraindicated treatment.
LEARNING POINTS
- Haemophilia A is characterized by frequent haemorrhage.
- Pharmacokinetic-guided personalized prophylaxis with Nuwiq® provided bleeding protection with good tolerability.
- Nuwiq® leads to a reduction of about 20% in both drug consumption and the annualized bleeding rate.
KEYWORDS
Haemophilia A, personalized prophylaxis, pharmacokinetics, coronary stent, recombinant FVIII, Nuwiq®
INTRODUCTION
Treatment of coronary events in patients with haemophilia is a challenge because of the need for antiplatelet therapy (which is essential to prevent stent thrombosis but increases haemorrhagic risk) and the lack of specific recommendations. It is generally accepted that patients with haemophilia should be treated in the same way as the general population. New-generation stents allowing short dual antiplatelet therapy together with P2Y12 receptor inhibitor administration are being used successfully. Nuwiq® is a fourth generation recombinant human FVIII produced in a human cell line by post-translational protein processing, including glycosylation and sulfation which closely mimic those of endogenous FVIII. It shows a glycosylation pattern similar to that of FVIII and so may be less immunogenic. Furthermore, Nuwiq® is fully sulfated at all tyrosine binding sites and has high binding affinity for VWF. As a consequence, it might be less likely to induce the development of FVIII inhibitors. Nuwiq® also has an extended circulating half-life in vivo compared with hamster cell line-derived recombinant FVIII products.
CASE DESCRIPTION
A 58-year-old patient with severe haemophilia A (HA) without inhibitors but with HCV and HIV seroconversion and undergoing plasma-derived replacement therapy, attended our centre in Catania, Italy, from 2016. In April 2017, he experienced an inferior acute myocardial infarction (AMI) and so underwent percutaneous coronary angioplasty with implantation of a bare metal stent (BMS). Haemorrhagic manifestations increased because of concomitant antiplatelet therapy and so the patient was moved to Nuwiq® in September 2017. He continued Nuwiq® with no bleeding episodes and had achieved a good clinical condition by April 2020, when he was hospitalized for an episode of rhabdomyolysis.
Methods and Procedures
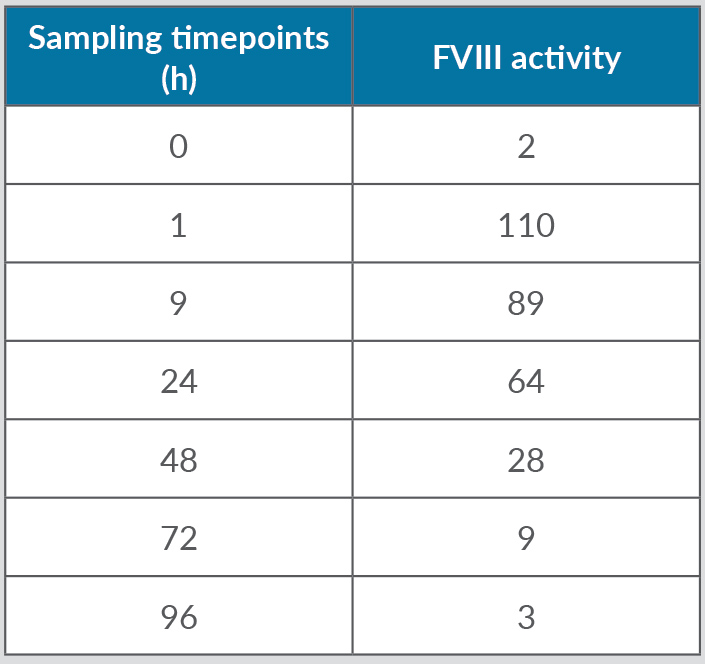
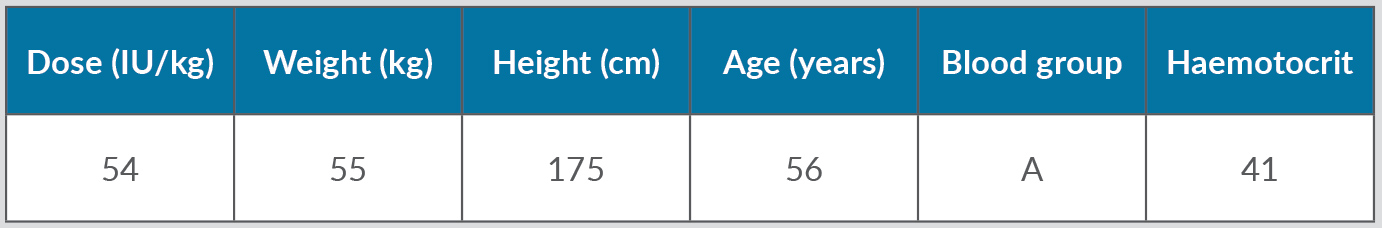
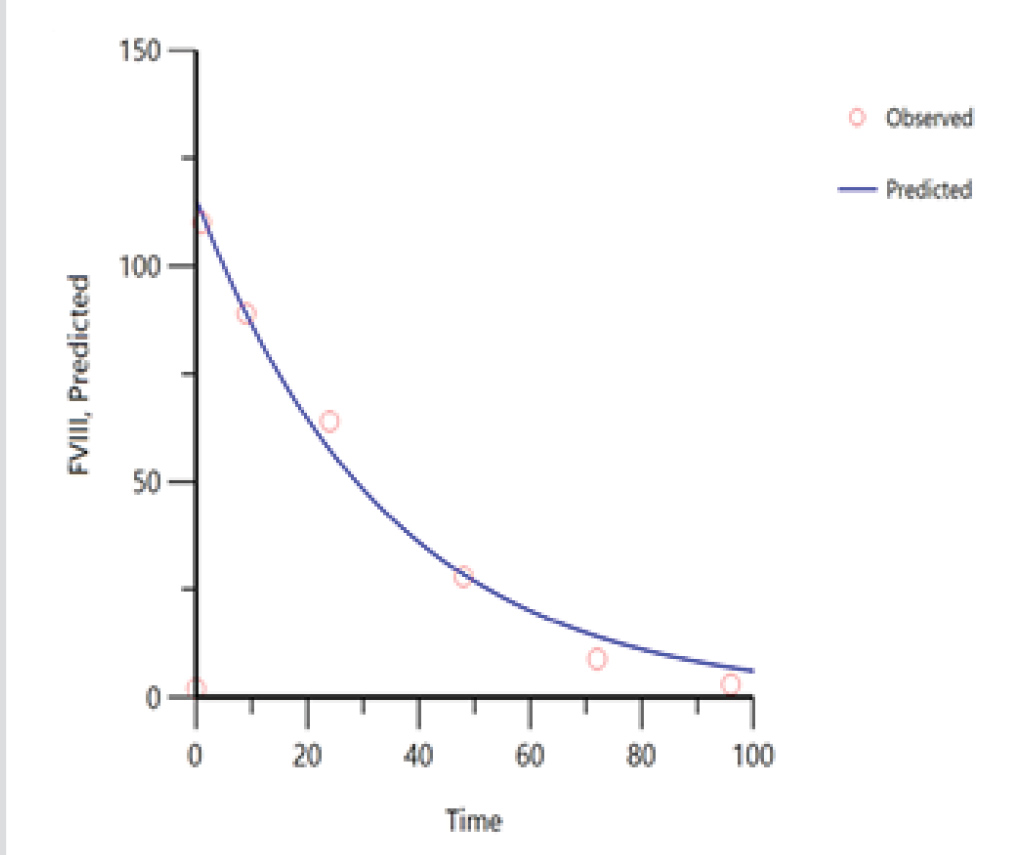
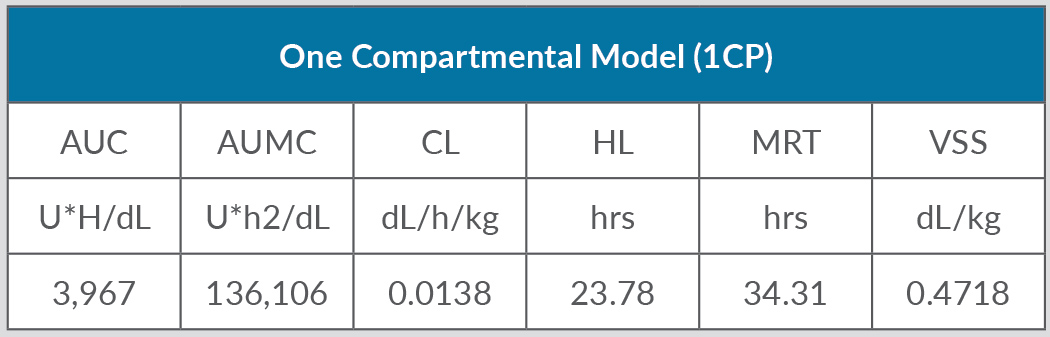
Despite replacement therapy given daily at a dose of 3,000 IU, the patient continued to experience recurrent haemarthroses (mainly in his knees, elbows and ankles) and poor control of bleeding (annualized bleeding rate (ABR) of 15). HIV and HCV were treated with dolutegravir and darunavir leading to the negativization of viral load as well as improvement in liver function indices. In April 2017, the patient experienced an inferior acute myocardial infarction (AMI) and so underwent a coronary angiography with percutaneous coronary angioplasty and bare metal stent (BMS) implantation, followed by double antiplatelet therapy with acetylsalicylic acid 100 mg/day and clopidogrel 75 mg/day. Despite replacement treatment with rFVIII, the number of haemorrhagic manifestations increased. However, withdrawal of antiplatelet therapy in the first months after AMI posed a very high risk of a new ischaemic episode due to intrastent thrombosis. Consequently, we decided to switch the patient to simoctocog alfa (Nuwiq®), a fourth generation factor VIII, believing that it would be more effective in preventing bleeding as it is manufactured in human embryonic kidney cell lines. As demonstrated by the NuPreviq study[1], the mean ABR during personalized prophylaxis with Nuwiq® was 1.45 for all bleeds, 0.79 for spontaneous bleeds, and 0.91 for joint bleeds, compared with standard prophylaxis. Moreover, during personalized prophylaxis, 83.1% of patients did not experience any spontaneous bleeds. In September 2017, our patient received Nuwiq® 3,000 IU per day. Due to the persistence of hemarthroses, related to the concomitant antiplatelet therapy, the dosage was reduced to 3,000 IU three times a week. A pharmacokinetic test showed a 3% activity level 96 hours after the last infusion (Tables 1 and 2, and Fig. 1). A reduction of about 20% in drug consumption compared with previous therapy and a reduction in ABR from 15 to 5 were documented. The patient maintained a good clinical condition until April 2020, when he was hospitalized for an episode of rhabdomyolysis, probably related to ongoing therapy with statins. During hospitalization, the patient continued Nuwiq® therapy, and no bleeding episodes were reported. Cardiac function also remained stable, despite a left ventricular ejection fraction of approximately 30%. Meanwhile, viral reactivation was observed, probably due to steroid use, so in November 2021 antiviral drugs were changed leading to negativization of the viral load. To date, the patient feels quite well with no bleeding episodes.
Table 1. Patient pharmacokinetics.
Table 2. Patient characteristics.
Figure 1. Pharmacokinetics of Nuwiq®. The concentrate shows monophasic decay, so the data fit with the one-compartment model but not with the two-compartment model.
Findings documented in vivo recovery within a normal range, very low clearance and a volume of distribution very similar to the theoretical value of plasma volume.
DISCUSSION
This case report shows that it is possible to effectively adjust replacement therapy in a patient on apparently contraindicated treatment. Our patient with severe HA underwent percutaneous coronary angioplasty with BMS implantation because of AMI. Nuwiq® appears to be well tolerated and effective in preventing bleeds, with a satisfactory pharmacokinetic profile. A pharmacokinetic test, which could be performed to offer patients personalized therapy[1], demonstrated a reduction of about 20% in drug consumption compared with previous therapy and a reduction in ABR (5 vs 15).