ABSTRACT
Drug-induced liver injury (DILI) is a heterogenous entity with a wide range of pathogenetic mechanisms and clinical manifestations. DILI is a diagnosis of exclusion. Metamizole (dipyrone) is an analgesic increasingly used in Europe, but there is limited information on its adverse effects. We report the case of a 56-year-old man with acute fever, malaise and general deterioration. Onset of symptoms occurred 12 hours after intake of metamizole for shoulder pain. The patient’s medical history was remarkable for three episodes of an inflammatory syndrome with hepatitis of unknown aetiology during the previous 3 years. However, retrospective enquiry showed each episode was preceded by metamizole intake shortly before symptom onset. Relevant differential diagnoses such as infection, vasculitis, autoimmune or metabolic diseases were excluded. Liver biopsy was compatible with DILI. Discontinuation of metamizole led to rapid clinical improvement and normalization of liver transaminases.
Metamizole is a very rare and poorly known cause of DILI with only a few published case reports in the literature. Careful medical history taking is important to identify the causative agent. Prompt recognition and discontinuation of the drug is crucial. Patients must be informed to avoid this medication in future.
LEARNING POINTS
- Metamizole is a rare cause of drug-induced liver injury (DILI).
- Taking a systematic medical and drug history is crucial for diagnosing DILI.
- DILI is a diagnosis of exclusion.
KEYWORDS
Metamizole, drug-induced liver injury (DILI), systemic inflammatory response syndrome
CASE DESCRIPTION
A 56-year-old man attended the emergency department in September 2019 with a 1-day history of fever, chills, malaise, nausea with vomiting, and general deterioration. Onset of symptoms occurred 12 hours after treatment with metamizole (dipyrone) which was initiated for presumed osteoarthritis of the right shoulder in a surgical outpatient clinic. The patient reported no use of other drugs, alcohol or recreational substances, and his travel history was unremarkable. He reported being intolerant to paracetamol, but not to other drugs. His medical history was remarkable for three previous similar episodes since 2016, each of which was attributed to a systemic inflammatory syndrome with hepatitis and eosinophilia. Despite extensive work-up to identify infectious, rheumatic or pharmacological reasons, no cause had been found.
Thorough retrospective re-evaluation of his drug history revealed that each episode was preceded by metamizole intake shortly before symptom onset. Co-medication with NSAIDs and ciprofloxacin was reported during the first and the second episode, respectively.
On admission, the patient was febrile (39.6°C) but haemodynamically stable. The rest of the physical examination was normal, there was no icterus and no rash.
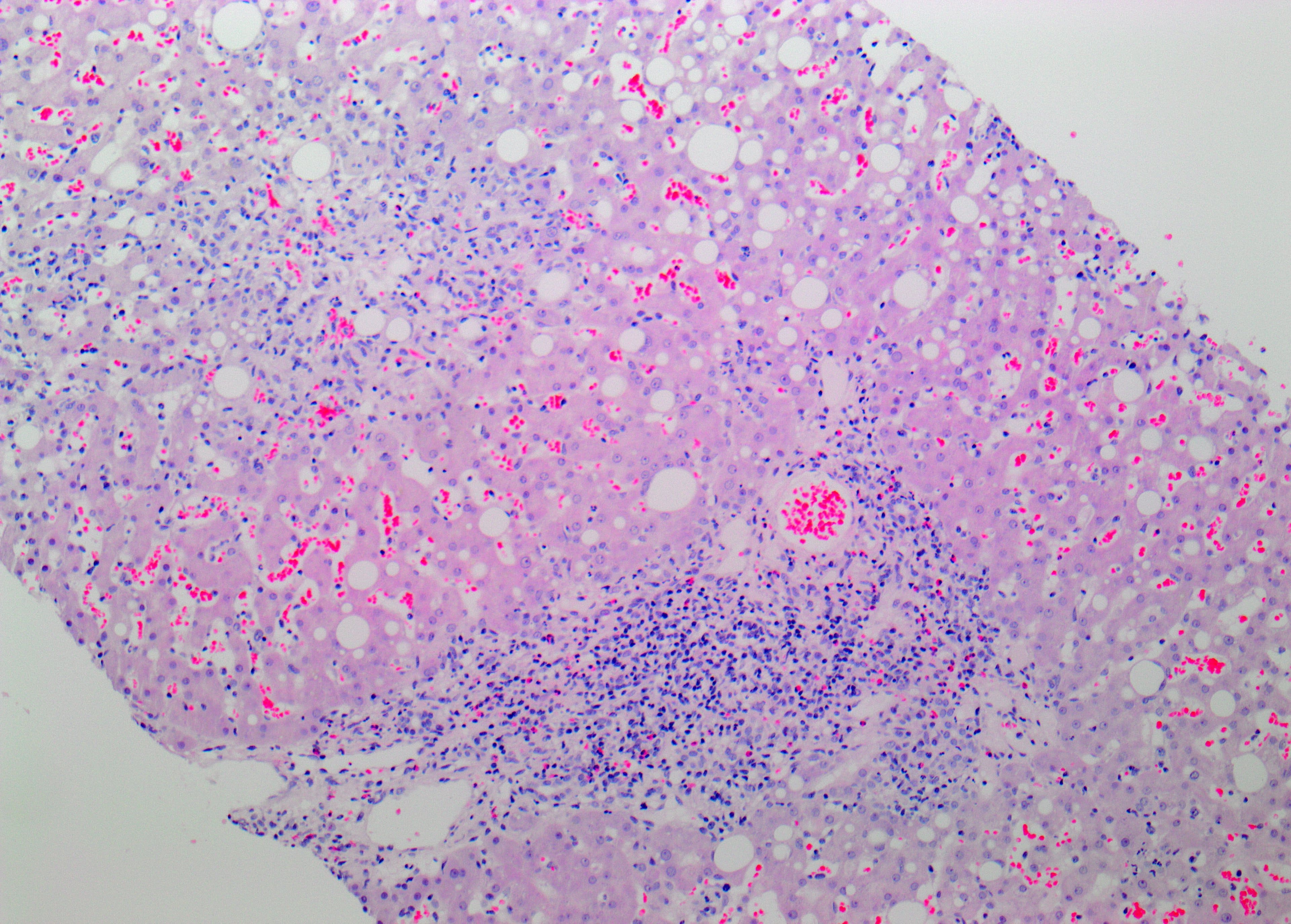
Laboratory tests showed an elevated white blood cell count (12.6×109/l) with neutrophilic predominance (91.7%, 12.2×109/l) and a low eosinophilic count (0.1%, 0.01×109/l) and elevated C-reactive protein (CRP) (132 mg/l). Liver enzymes were markedly elevated: alanine aminotransferase (ALT) 1,374 U/l, aspartate transaminase (AST) 804 U/l and alkaline phosphatase (ALP) 51 U/l. The R factor (ALT/upper limit of normal (ULN)/ALP(ULN) was 43, suggesting hepatocellular damage [1]. Bilirubin, albumin and INR were within normal limits. Blood and urine cultures were negative. Abdominal ultrasound showed hepatic steatosis with no other abnormalities. Viral hepatitis (A, B, C and E) and autoimmune hepatitis were excluded. Cytomegalovirus (CMV), Epstein–Barr virus (EBV) and human immunodeficiency virus (HIV) as well as haemochromatosis and Wilson disease were also excluded. The liver biopsy revealed subacute perivenular hepatocyte necrosis as well as a predominantly eosinophilic inflammatory infiltrate suggestive of DILI (Fig. 1). The exact cause could not be identified, however according to the drug history, metamizole appeared to be the most likely causative agent.
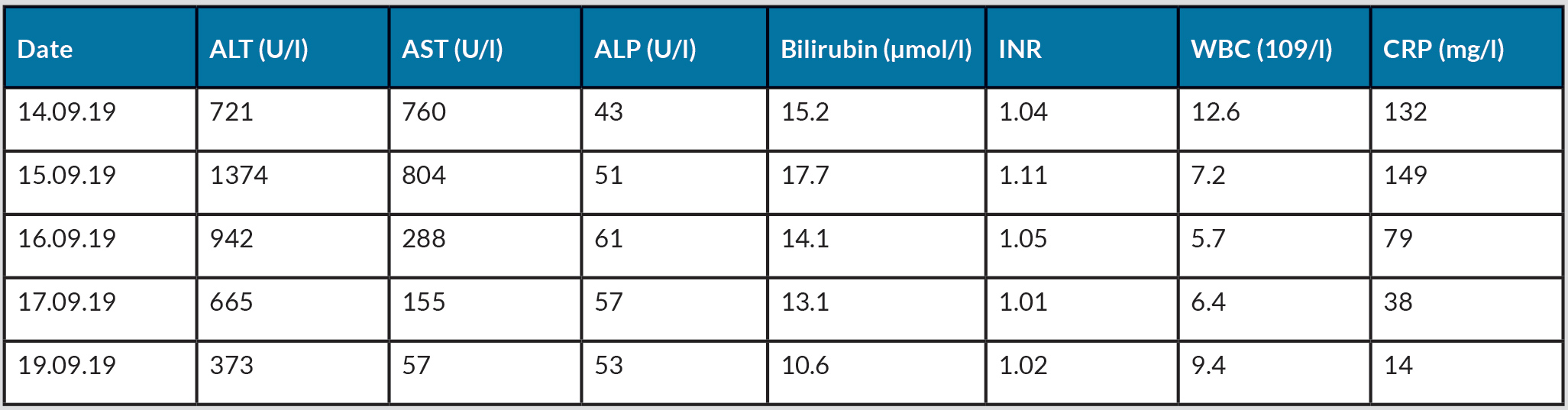
Management consisted of immediate withdrawal of metamizole, intravenous fluid administration and initial empirical antibiotic treatment with amoxicillin/clavulanic acid until a bacterial infection could be excluded. The clinical course was favourable. Within 3 days, the fever disappeared, the patient’s general state significantly improved and the transaminases decreased (Table 1), consistent with a positive dechallenge. The patient was successfully discharged 7 days after admission with the advice to strictly avoid metamizole.
Figure 1. Histology of liver biopsy shows perivenular accentuated hepatocyte necrosis and eosinophil-rich portal inflammatory infiltrate
Table 1. Evolution of laboratory values during hospitalization
DISCUSSION
The temporal correlation between metamizole intake and the occurrence of clinical symptoms and elevated liver enzymes in four different episodes, including one observed dechallenge, makes the diagnosis of metamizole-induced liver injury very likely in our case.
DILI should be considered in patients with acute hepatitis without obvious aetiology [2,3]. It is a diagnosis of exclusion and can be difficult to detect, especially when the presentation resembles a systemic inflammatory syndrome, as in our case. It is important to rule out other causes of elevated liver enzymes with systemic symptoms, for example, infection and autoimmune or metabolic disorders. Careful medical history taking is crucial to identify DILI, as in our case, where it revealed a correlation between metamizole intake and the onset of liver injury. Rapid recovery after stopping (dechallenge) and re-occurrence after re-exposure to the suspect drug (rechallenge) might provide further support and confirm the diagnosis of DILI. Because of the lack of specific biomarkers or diagnostic tests, causality assessment scores, like for instance RUCAM (Roussel Uclaf Causality Assessment Method), can help assess the probability of DILI [4,5]. The severity of DILI is graded by the measurement of transaminases, bilirubin, INR, and clinical factors indicating liver failure. Patients may present with a broad variety of symptoms and organ involvement.
Our patient experienced four episodes of a systemic inflammatory syndrome with markedly elevated liver enzymes. Extensive medical work-up never identified an infection or a rheumatological or haematological cause. However, the medical history, laboratory and histological findings were suggestive of first grade (mild) DILI. The RUCAM score was 11. In all four episodes, metamizole had been taken hours to several days before the onset of symptoms. DRESS syndrome (Drug Rash with Eosinophilia and Systemic Symptoms) seemed to be less likely due to the lack of rash and eosinophilia, and the absence of the typical causative drugs (such as antiepileptics, allopurinol and antibiotics).
Metamizole, or dipyrone, is a widely used non-opioid analgesic drug with spasmolytic and antipyretic properties [3,5]. It has a good analgetic effect with fewer gastrointestinal and cardio-renal adverse events compared with NSAIDs. However, agranulocytosis is a rare and serious adverse drug reaction with a potentially fatal outcome. Therefore, metamizole is banned in some countries, including the USA and UK [5]. The drug undergoes extensive hepatic metabolism mediated by cytochrome P450 (CYP) and other enzymes. Therefore, drug-related hepatotoxicity as well as drug–drug interactions are possible and induction of CYP enzymes by metamizole has recently been described [6]. There are only a few publications (mostly case reports) on the possible hepatic adverse effects of metamizole. The pathomechanism remains unclear, however some reports suggest immunoallergic (idiosyncratic) mechanisms [4,5].
CONCLUSION
Our presumed diagnosis was metamizole-induced liver injury. DILI is a diagnosis of exclusion and can be difficult to detect, especially when it mimics a systemic inflammatory syndrome, as in our case. Careful medical history taking was crucial to identify the association between metamizole intake and the onset of liver injury.
Metamizole is a very rare and poorly recognised cause of severe DILI with inflammatory syndrome, with only a few published case reports in the literature.