ABSTRACT
Clot in transit (CIT) is a rare condition in which a venous thromboembolism becomes lodged in the right heart. It is seen in up to 18% of patients with massive pulmonary embolism, and if left untreated, mortality rates are between 80% and 100%. The identification and management of CIT are crucial. However, there are no current guidelines for the treatment of CIT. We present the case of a 44-year-old woman who was found to have CIT that was ultimately treated with medical management.
LEARNING POINTS
- Clot in transit (CIT) is a dangerous entity that must be promptly managed.
- Risk factors for CIT include a history of heart failure, a pre-existing central venous catheter and recent hospitalization.
- New interventions are emerging for the treatment of CIT.
KEYWORDS
Clot in transit, pulmonary embolism, venous thromboembolism, thrombectomy
INTRODUCTION
Clot in transit (CIT) is a rare and dangerous entity defined as a ‘venous thromboembolism (VTE) that has become lodged in the right heart’[1] before arriving at the pulmonary vasculature. It can be seen in 3% of patients with pulmonary embolism (PE), and up to 18% of those with massive PE. Mortality rates are between 80% and 100% in those with untreated CIT, and far higher in patients with PE and CIT than in those without CIT [1,2]. Interestingly, it is unclear whether CIT is a marker of disease severity or contributes to mortality by itself [2]. Clot characteristics such as attachment, mobility and location are not associated with changes in mortality [1]. CIT is best diagnosed with an echocardiogram, which allows visualization of the CIT and other intracardiac thrombi. Echocardiograms are performed in those with PE to evaluate for right heart dysfunction. Due to the rarity of the condition, there are no current guidelines for the treatment of CIT. ‘Knowledge is limited to case reports, small case series, and meta-analyses that offer conflicting recommendations’ [1]. Treatment options include anticoagulation, systemic thrombolysis, surgical embolectomy, and recently approved percutaneous catheter thrombectomy, although treatment decisions can be complicated by a patient’s comorbidities. We present the case of a 44-year-old woman who was found to have CIT that was ultimately treated with medical management.
CASE DESCRIPTION
The patient was a 44-year-old woman from the Dominican Republic with a history of hypertension, obesity class I, protein S deficiency with inferior vena cava thrombus, and PE who was referred by her cardiologist to the emergency room due to a 3-day history of sudden onset shortness of breath, associated with retrosternal chest pain and aggravated by breathing.
The patient reported compliance with apixaban 5 mg twice daily at home. Her surgical history was significant for an appendectomy and a caesarean section. Her mother had a history of myocardial infarction, and she was aware of the clotting disorder in her family. On examination, she was afebrile, heart rate was 82 bpm, blood pressure was 135/91 mmHg, and saturation was 97% on room air. A chest x-ray did not show any acute cardiopulmonary abnormality. A CT angiogram of the chest revealed acute PE with complete occlusion of the tertiary right lower lobe branches. There was a 1.5×1.4 cm pleural-based density in the right lower lobe that was read as either focal atelectasis or Hampton's hump secondary to PE or neoplasm. An electrocardiogram showed normal sinus rhythm with left ventricular hypertrophy.
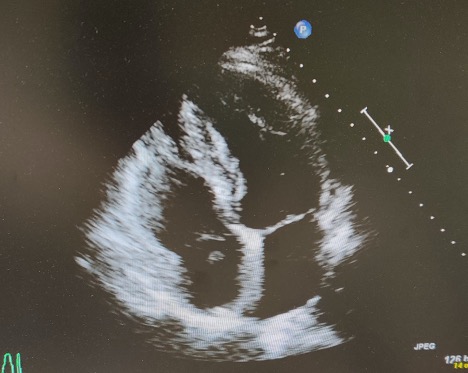
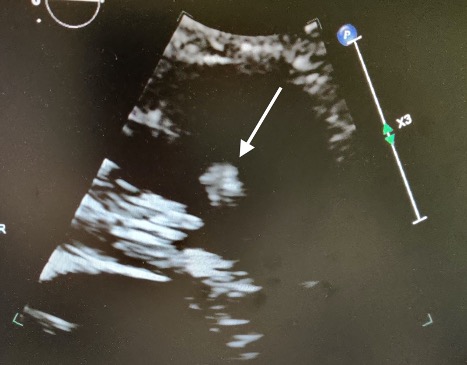
The patient was started on low molecular weight heparin (LMWH) and bridged with warfarin for a goal INR of between 2 and 3. Ultrasound of the bilateral lower extremities was negative for deep venous thrombosis (DVT). Serial troponin and brain natriuretic peptide (BNP) were normal. An echocardiogram showed a mobile mass oscillating between the right atrium and the right ventricle (RV), representing a CIT (Fig. 1). There was no evidence of right heart dysfunction on echo. Interventional radiology was consulted and after discussion with cardiology, a decision was made to continue anticoagulation and repeat the echocardiogram. Repeat echocardiogram was notable for an echo dense, oscillating mass in the right ventricular outflow tract adjacent to the pulmonary valve with normal RV function (Fig. 2). The patient was subsequently transferred to a tertiary care centre for further intervention.
Figure 1. Initial echocardiogram showing a mass between the right atrium and right ventricleventricular outflow tract
Figure 2. Repeat echocardiogram showing a mass near the right ventricular outflow tract
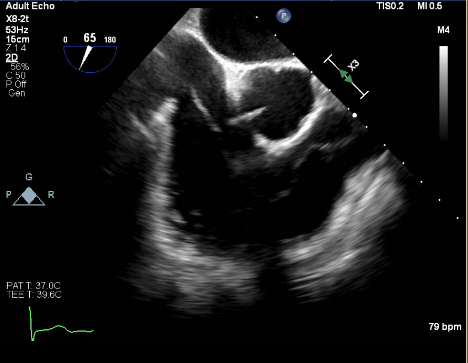
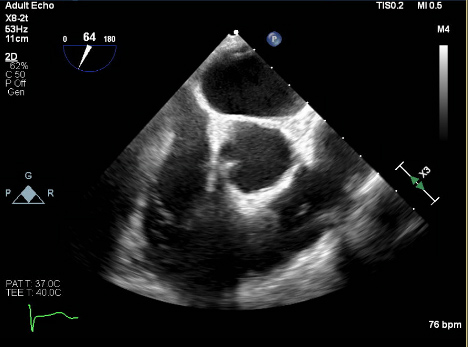
At the tertiary care centre, the patient was admitted to telemetry and a STAT transthoracic echocardiogram was performed confirming a linear mobile density in the right ventricle measuring roughly 3.7 cm. This density was seen moving in and out of the right atrium. A transesophageal echocardiogram (TEE) was performed the following day and showed a mobile, long, thin mass visualized extending from the inferior vena cava junction into the right ventricular outflow tract (Fig. 3). A large, bulkier portion measuring over 4 cm rested just before the pulmonary valve (Fig. 4). TEE confirmed transit to the right ventricle and this mass was deemed highly consistent with a thrombus-in-transit.
Interventional radiology was then consulted for thrombectomy. An interdisciplinary discussion was held and the decision was made to abort thrombectomy and proceed conservatively with medical management due to the risks of the procedure in the context of the patient’s haemodynamic stability and lack of RV strain. The patient was started on IV heparin while on coumadin bridging for an INR goal of 2–3. Once the patient was completely bridged to warfarin, she was deemed medically stable for discharge with close haematology follow-up.
Figure 3. Transesophageal echocardiogram showing a large thrombus present at the inferior vena cava junction traveling into the right ventricle with a distal, thicker segment by the pulmonary valve
Figure 4. Transesophageal echocardiogram showing an enhanced view of the bulkier, thicker distal segment of the clot resting in the right ventricular outflow tract by the pulmonary valve
DISCUSSION
Up to 600,000 cases of VTE occur each year in the USA [3]. VTE is an entity comprising deep vein thrombosis (DVT) and PE.
Risk factors for DVT, and thus PE, are inherited and acquired components of Virchow’s triad [1,3]. Clinically, patients with PE can be asymptomatic or in cardiogenic shock, depending on the size and location of the embolus. The most common symptom is dyspnoea. PE is best diagnosed with a CT pulmonary angiogram.
PE can be classified into low, intermediate and high-risk categories, with risk guiding the treatment [4]. Low-risk patients are those without evidence of end-organ damage or haemodynamic instability. These patients can be managed with anticoagulation. Intermediate-risk patients are those with end-organ damage but haemodynamically stable. High-risk patients are those who present with signs of haemodynamic instability and are at risk for sudden death [4,5]. Intermediate and high-risk patients often require additional treatments to decrease the clot burden. Options include systemic intravenous thrombolysis, surgical thromboembolectomy and catheter thromboembolectomy. These three treatments may not be available at all centres and are subject to centre expertise. Intracerebral haemorrhage is a feared complication of systemic intravenous thrombolysis [6]. The goal of treatment is to reduce clot burden, prevent recurrent VTE, and prevent chronic thromboembolic pulmonary hypertension [3,6].
There are three types of right heart thrombus, types A, B and C [1]. Type A refers to CIT. Additionally, thrombi in the superior or inferior vena cava, and those stuck in a patent foramen ovale (PFO), fall into this class [7]. Morphologically, these thrombi are described as serpentine, and they may be free floating or have a thin form of attachment [1,8]. All of our patient’s echocardiograms met these criteria. Type B right heart thrombus refers to mural thrombi, which are attached to either the right atrium or right ventricle. These thrombi are thought to have formed in situ, and have lower mortality rates than type A thrombi. Finally, type C thrombi are those that share morphology with cardiac myxoma.
Several risk factors for the development of CIT have been identified. In a case series of 665 PE patients comparing those with and without CIT, Garvey et al. found that patients with CIT were more likely to have a history of heart failure, a pre-existing central venous catheter and recent hospitalization [9]. It is hypothesized that heart failure contributes to CIT because it promotes stasis in the right heart that entraps the CIT. This study had other notable findings as well. Compared with patients without CIT, those with CIT more often presented with haemodynamic instability and a change in mental status. They had higher 7-day mortality rates and higher rates of intubation. Our patient lacked these risk factors, but did have known protein S deficiency contributing to a hypercoagulable state.
There are currently no treatment guidelines for CIT. However, although a patient might have a low-risk PE, the presence of CIT should escalate them to the high-risk category due to the risk of decompensation [4,10]. Thus, even though the CIT has been treated with anticoagulation only [11], patients should receive other treatments and ICU level care. It is acceptable for mild PE to be treated with anticoagulation only [5]. For intermediate-risk PE, high-risk PE and CIT patients, other treatment modalities as mentioned earlier should be implemented, although there are special considerations in CIT. For example, systemic thrombolysis has been used for the treatment of PE and CIT. For CIT however, there is a risk of uneven dissolution of the thrombus and embolization of what remains [1]. If systemic thrombolysis does not show resolution of the CIT by 24 hours, then surgery may be considered. Low-dose tissue plasminogen activator (TPA) as shown in the Moderate Pulmonary Embolism Treated with Thrombolysis trial has been used for the treatment of CIT [2,6]. Additionally, if the CIT is stuck in a PFO, surgical embolectomy is preferred over systemic thrombolysis because there is an increased risk of paradoxical embolus. The downside to surgical embolectomy is the need for cardiopulmonary bypass, which may pose too high a risk in a haemodynamically unstable patient. A pooled analysis of 328 patients with right heart thrombus treated with either anticoagulation, systemic thrombolysis or surgical embolectomy found that ‘thrombolysis and surgical embolectomy are both effective strategies, with a slightly increased probability of survival with thrombolysis’ [12]. Finally, two new endovascular treatment options have emerged. First, catheter-based mechanical aspiration thrombectomy using the FlowTriever device has been shown to be effective for the treatment of CIT [13,14]. This treatment recently (January 2021) received FDA approval for CIT, and has the benefit of not requiring cardiopulmonary bypass. A different endovascular device called the AngioVac System has been used in 47 patients with right heart thombi [15]. This is a vacuum-assisted thrombectomy device with a comparatively large aspiration cannula that offers good safety and efficacy, although it does require extracorporeal bypass (often <1 hour).
CONCLUSION
CIT is a rare entity in which a venous thromboembolism has become lodged in the right heart on its way to the pulmonary artery. It is associated with concurrent PE and has a higher mortality than PE alone. Although cases have been treated with anticoagulation only, high mortality rates should prompt treatment escalation using one of the options described. That being said, multidisciplinary discussions should always be had with patients to involve them in their care.