ABSTRACT
Dual anti-neutrophil cytoplasmic antibody-associated vasculitis (AAV) characterized by the presence of both anti-proteinase-3 (PR3-ANCA) and anti-myeloperoxidase (MPO-ANCA) antibodies is a rare clinical entity. Only few cases have been reported previously, most of which were associated with infections, drugs, autoimmune diseases and malignancies. Herein, we describe a young woman who presented with rapidly progressive glomerulonephritis with hypocomplementemia and markedly elevated anti-PR3 and anti-MPO titres. Meticulous work-up ruled out all possible secondary causes. Renal biopsy showed the presence of focal fibrocellular crescents with focal mesangial hypercellularity. Immunofluorescence and electron microscopy showed pauci-immune deposits. The patient was treated with an induction regimen comprising oral prednisolone and cyclophosphamide. She attained both clinical and serological remission at 3 months and is currently on an azathioprine-based maintenance regimen. We have extensively reviewed all previous cases of dual AAV and have formulated an approach to diagnose and treat this rare entity.
LEARNING POINTS
- Dual anti-neutrophil cytoplasmic antibody-associated vasculitis characterized by both PR3-ANCA and MPO-ANCA antibodies is a rare clinical entity.
- Prior to treating with immunosuppression, we need to rule out secondary aetiologies such as drugs, certain infections, autoimmune diseases and haematological malignancies.
- Atypical presentations such as hypocomplementemia, other serological abnormalities like positive ANA, cryoglobulins, anti-histone antibody and histology showing mesangial hypercellularity, interstitial inflammation and lack of pauci-immunity, may create a diagnostic dilemma.
KEYWORDS
ANCA-associated vasculitis, anti-proteinase-3, anti-myeloperoxidase, hypocomplementemia, crescents
INTRODUCTION
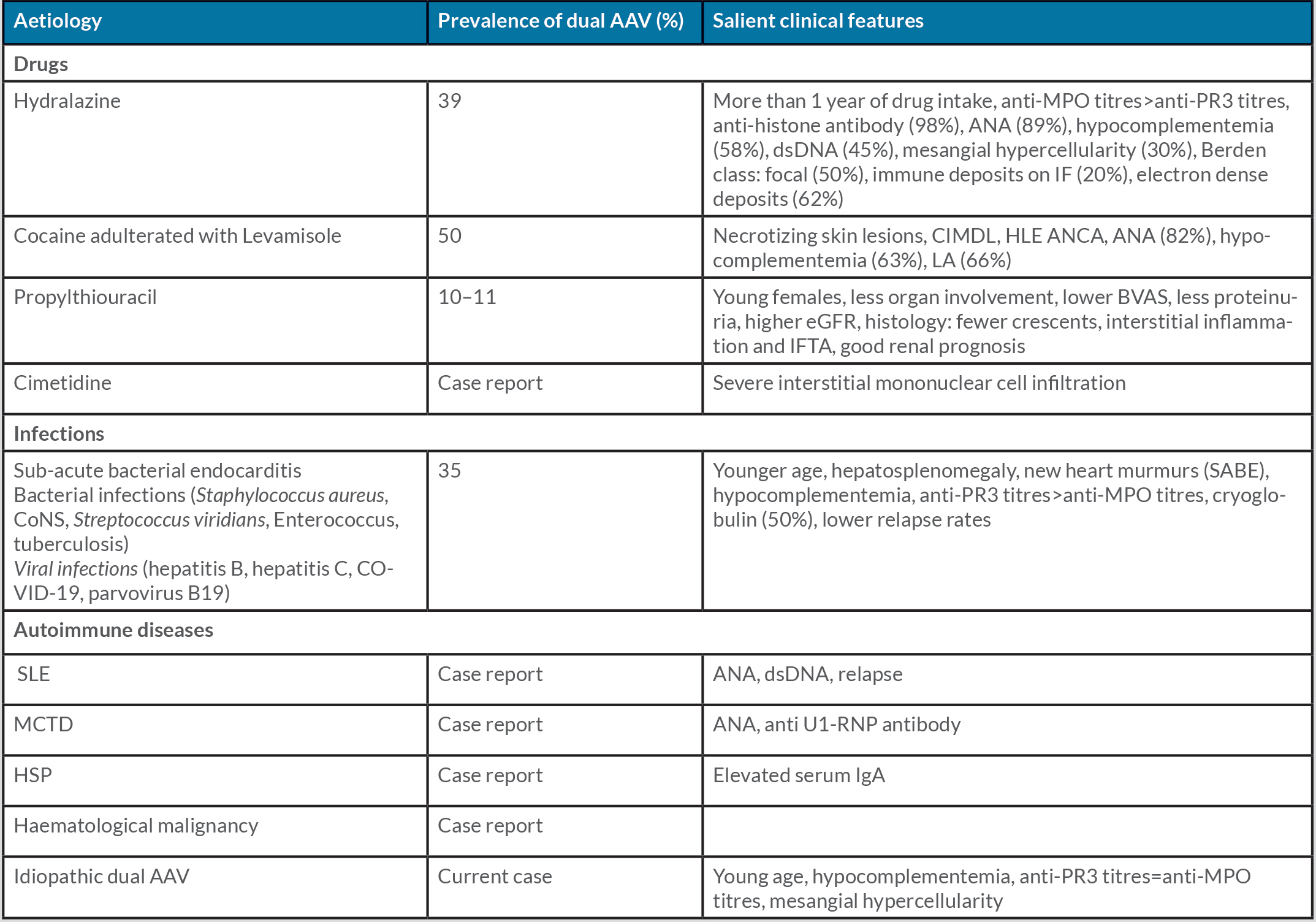
Anti-neutrophil cytoplasmic antibody (ANCA)-associated vasculitis (AAV) is a small vessel vasculitis associated with either anti-proteinase-3 (PR3-ANCA) or anti-myeloperoxidase (MPO-ANCA) antibodies. PR3-ANCA is the predominant antibody seen in granulomatosis with polyangiitis (GPA), whereas MPO-ANCA is the dominant antibody in microscopic polyangiitis (MPA), eosinophilic granulomatosis with polyangiitis (EGPA) and renal limited AAV [1]. The presence of both PR3-ANCA and MPO-ANCA is unusual and has been previously reported with certain infections, drugs, autoimmune diseases and malignancies [2–5]. Herein, we report a rare case of idiopathic renal limited dual AAV which was treated successfully with immunosuppression.
CASE DESCRIPTION
A 30-year-old Indian woman presented with history of arthralgia of 2.5 years’ duration, swelling of the feet for 1 month and oliguria of 7 days’ duration. There was no history of any skin lesions, photosensitivity, hair loss, ear or nasal discharge, cough, haemoptysis, abdominal pain, melena, or wrist or foot drop. On examination, the patient was found to be hypertensive with elevated jugular venous pulsations and pitting pedal oedema. Coarse crepitations were heard on auscultation of basal lung fields.
Her initial laboratory parameters were as follows: haemoglobin 5.1 g/dl, total WBC count 11,100 cells/mm³, platelet count 3.4×105cells/mm³, serum creatinine 6.87 mg/dl, and albumin 2.8 g/dl. Urine microscopy revealed protein 1+, RBC 47/HPF (dysmorphic RBC 60% with no RBC casts), WBC 11/HPF, and 24-hour urine protein was 1.6 g/day. Ultrasound of the abdomen showed normal sized kidneys and increased echogenicity with maintained corticomedullary differentiation. Autoimmune and serological work-up findings were as follows: ANA negative, dsDNA 12 U/ml, C3 29.7 (90–180 mg/dl), C4 9 (10–40 mg/dl), antiphospholipid antibody (APLA) panel negative, anti GBM antibody negative, anti U1-RNP antibody negative, anti histone antibody negative, viral serology (hepatitis B and C) negative, cryoglobulin negative, PR3-ANCA >200 U/ml and MPO-ANCA >200 U/ml.
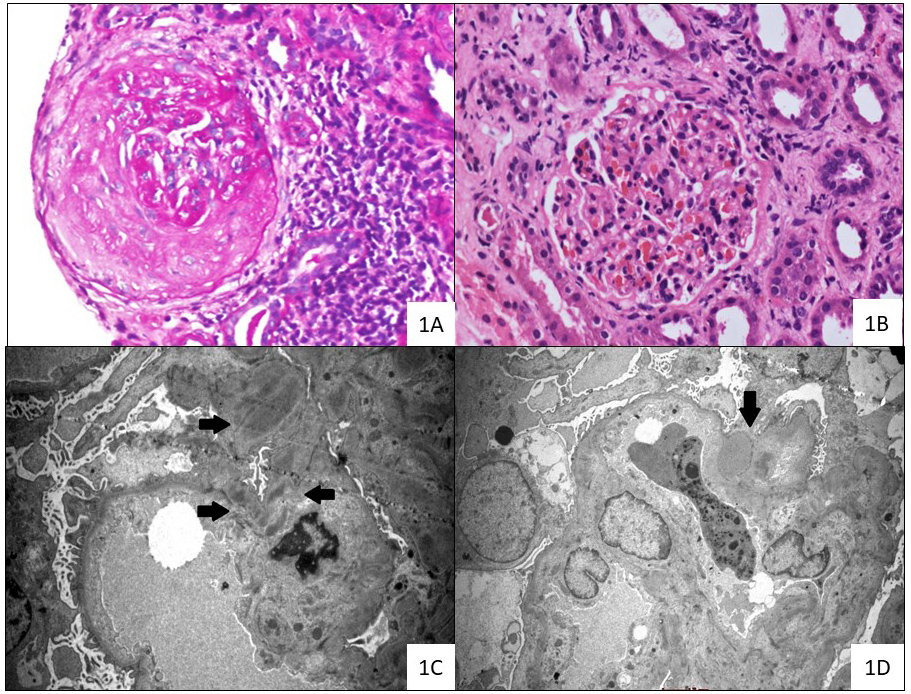
The patient had no history of intake of any drugs known to cause ANCA vasculitis (Table 1). A thorough infective work-up did not isolate any pathogenic organisms. The findings included sterile blood and urine culture, normal procalcitonin levels, negative COVID-19 PCR and absence of vegetations on echocardiography. The patient underwent a kidney biopsy with three cores sent for light microscopy (LM), immunofluorescence microscopy (IF) and electron microscopy (EM). Of the 10 glomeruli available for evaluation on LM, four were globally sclerosed, two showed the presence of fibrocellular crescents (Fig. 1A and the remaining viable glomeruli showed mild mesangial expansion with focal segmental mesangial hypercellularity (Fig. 1B). There was no evidence of fibrinoid necrosis, endocapillary hypercellularity or capillary wall reduplication. The tubulointerstitium showed diffuse acute tubular injury with moderate patchy interstitial fibrosis and tubular atrophy (40%) with mononuclear cell infiltration. On IF, there was granular mesangial staining for IgG (1+), C3 (2+), Kappa (1+) and Lambda (1+). Ultrastructural evaluation showed a few mesangial, sub-endothelial and sub-epithelial deposits (Fig. 1C,D).
Figure 1. Renal biopsy findings of the current case of dual ANCA-associated vasculitis
(1A) Fibrocellular crescent occluding capillary loop with interstitial mononuclear cell infiltration (haematoxylin and eosin stain; original magnification ×200).
(1B) Glomerulus with congested loops and mild mesangial hypercellularity (periodic acid-Schiff stain; original magnification ×200).
(1C) Glomeruli demonstrating mesangial ill-defined electron-dense deposits (bold black arrows) (transmission electron microscope; original magnification ×4200).
(1D) Segment of glomeruli displaying a rare para-mesangial notch hump-like electron-dense deposit (marked with bold black arrow). Overlying podocytes showed marked foot process effacement. (transmission electron microscope; original magnification, ×4200)
Table 1. Causes of dual anti-neutrophil cytoplasmic antibody-associated vasculitis
AAV, anti-neutrophil cytoplasmic antibody-associated vasculitis; ANA, anti-nuclear antibody; BVAS, Birmingham Vasculitis Activity score; CIMDL, cocaine-induced midline destructive lesions; CoNS, coagulase negative staphylococcus; dsDNA, double-stranded DNA; eGFR, estimated glomerular filtration rate; HLE, human leucocyte elastase; HSP, Henoch-Schonlein purpura; IF, immunofluorescence; IFTA, interstitial fibrosis and tubular atrophy; LA, lupus anticoagulant; MCTD, mixed connective tissue disease; MPO, myeloperoxidase; PR3, proteinase 3; SABE, sub-acute bacterial endocarditis; SLE, systemic lupus erythematosus.
The patient was treated with an induction regimen of oral prednisolone (1 mg/kg/day) and a renal adjusted dose of oral cyclophosphamide. Her symptoms improved within a month. Her parameters at a follow-up visit at 3 months were as follows: serum creatinine 0.8 mg/dl, Hb 10.5 g/dl; urine microscopy: protein negative, RBC 16/HPF, WBC 4/HPF, 24-hour protein 0.37 g/day, PR3-ANCA 61 U/ml, MPO-ANCA 133 U/ml, C3 115 mg/dl and C4 28.7 mg/dl. Steroid was tapered and azathioprine was initiated as maintenance agent. The patient was continuing to do well at her last visit (12 months) with normal renal function and negative ANCA titres.
DISCUSSION
The simultaneous occurrence of both PR3-ANCA and MPO-ANCA in patients with vasculitis is an uncommon phenomenon. In most cases, there seems to be a triggering factor such as drugs, infections, malignancy or autoimmunity (Table 1). Drug metabolites can bind to both MPO and PR3 antigens expressed by neutrophils and can induce an antigenic change predisposing to antibody production. An alternate hypothesis is drug-mediated neutrophil apoptosis with the formation of apoptotic blebs expressing MPO and PR3 antigens leading to ANCA production [3]. A unique form of neutrophil cell death is NETosis, wherein neutrophils release decondensed chromatin and granular contents into extracellular space forming a sticky mesh-like network containing MPO and PR3 antigens. Antigen-presenting cells will pick up these antigens from the neutrophil extracellular traps (NETs), thus activating autoreactive T and B cells [6]. Infections are known to trigger ANCA formation, predominantly PR3-ANCA. Stimulation of peripheral blood mononuclear cells isolated from patients with ANCA vasculitis with unmethylated CpG oligonucleotide sequences found in bacterial and viral DNA resulted in increased production of ANCA (PR3>MPO-ANCA) [7]. The presence of dual ANCAs in autoimmune disease can be explained by the phenomenon of epitope spreading, wherein after the formation of an autoantibody, the antibody response may generalize to the rest of the auto antigens [6].
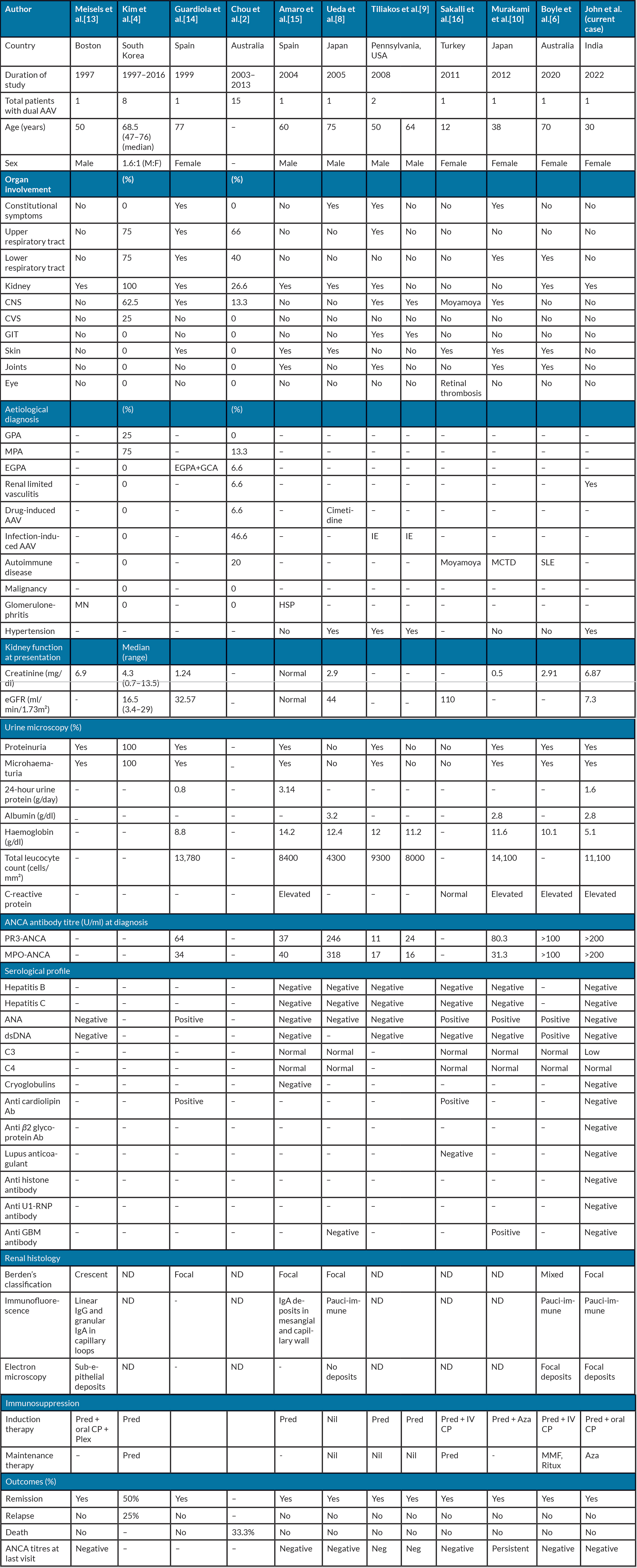
Table 2 lists previously reported cases of dual AAV [2,4,6,8–10]. In our case, we meticulously ruled out all possible secondary causes of dual AAV as given in Table 1. The clinical pointers which may enable an aetiological diagnosis to be narrowed down are the presence of extra-renal involvement, ANCA titres, associated serological abnormalities, complement levels, atypical histology like mesangial hypercellularity, immune deposits and interstitial inflammation. The presence of cutaneous gangrene and midline destructive lesions such as nasal septal perforation, palatal perforation or orbital erosive lesions, should lead to a strong suspicion of cocaine addiction [11]. Hepatosplenomegaly and new heart murmurs are pointers to sub-acute bacterial endocarditis (SABE) [5,9]. Drug-induced AAV is associated with markedly elevated anti-MPO antibody titres more than anti-PR3 titres [3,11], whereas in infections, PR3 titres are elevated more than MPO titres [5]. Dual AAV with marked elevation of both PR3 and MPO titres is seen in autoimmune [6] and idiopathic aetiologies, as in our case. Human leucocyte elastase (HLE) ANCA is specifically associated with cocaine-induced midline destructive lesions [11]. Associated serological abnormalities can predict underlying aetiology: anti-histone antibody (hydralazine-induced lupus nephritis) [3], anti U1-RNP antibody (mixed connective tissue disease) [10] and cryoglobulinemia (SABE, HCV infection or haematological malignancies) [5].
There is growing evidence of the involvement of an alternate complement pathway in the pathogenesis of AAV. Hypocomplementemia (low serum C3) is seen in 13–35% of patients with AAV. Patients with low C3 AAV are older, have higher mortality rates, have more severe tubulointerstitial injury on renal histology, and are more likely to progress to kidney failure [12]. Hypocomplementemia is a common feature seen in all aetiologies of dual AAV (Table 1), but whether this will translate to poor outcomes can be answered only after long follow-up studies. However, our patient achieved remission despite having hypocomplementemia, severe renal dysfunction and proteinuria at presentation and renal histology showing moderate to severe tubulointerstitial injury with C3 deposits on IF. Mesangial hypercellularity and prominent interstitial inflammation are pointers to drug-induced AAV [3,11], whereas immune deposits on IF are commonly seen with infections [5].
CONCLUSION
Dual AAV is a rare form of small vessel vasculitis which is associated with certain drugs, infections, autoimmune diseases and haematological malignancies. The majority of patients in published reports attained clinical and serological remission following immunosuppressive therapy. Future studies are needed to evaluate the underlying pathogenic mechanisms as well as long-term renal outcomes.
Table 2. Comparison of previously reported cases of dual anti-neutrophil cytoplasmic antibody associated vasculitis with the current case
AAV, anti-neutrophil cytoplasmic antibody-associated vasculitis; Ab, Antibody; ANA, anti-nuclear antibody; Aza, azathioprine; CNS, central nervous system; CP, cyclophosphamide; CVS, cerebrovascular system; dsDNA, double-stranded DNA; EGPA, eosinophilic granulomatosis with polyangiitis; GBM, glomerular basement membrane; GCA, giant cell arteritis; GIT, gastrointestinal tract; HSP, Henoch-Schonlein purpura; IE, infective endocarditis; MCT, mixed connective tissue disease; MN, membranous nephropathy; MPO, myeloperoxidase; ND, not done; PR3, proteinase 3; Pre, prednisolone; Plex, plasmapheresis; Ritux, rituximab; RNP, ribonucleoprotein; SLE, systemic lupus erythematosus.