ABSTRACT
Splenic infarction can occur as an infrequent thrombotic manifestation in polycythaemia vera (PV) and is usually catastrophic. We describe the case of a middle-aged woman who was diagnosed with PV 3 months before she presented to the emergency department with acute limb ischaemia. A splenic infarction detected on diagnostic imaging during her hospital stay was treated conservatively with modification of her hydroxyurea dose along with pain management, without the need for surgery.
LEARNING POINTS
- Multiple splenic infarctions are an uncommon presentation in polycythaemia vera.
- Patients can present with vague abdominal pain, so a high index of suspicion is necessary and diagnosis is usually radiological.
- Splenic infarction in polycythaemia patients can be treated successfully with hydroxyurea and pain management with aspirin.
KEYWORDS
PV, polycythemia vera, infarction, spleen
INTRODUCTION
Polycythaemia vera (PV) is a myeloproliferative disorder characterized by the proliferation of all three (erythroid, megakaryocytic and granulocytic) cell lines. The causative mutation is in the JAK2 tyrosine kinase gene [1]. Many patients are initially asymptomatic but later may develop complications of this disease, including arterial and venous thrombosis in different sites, including the spleen [2, 3]. Increased blood thickness and decreased blood flow contribute to an elevated risk of thrombosis [4]. Another clinical manifestation is splenomegaly due to extramedullary haematopoiesis. Splanchnic vein thrombosis (SVT) and splenic infarction with splenomegaly are infrequent but critical complications of myeloproliferative neoplasms (MPN) [5, 6].
We describe a patient with recently diagnosed PV who had gradual onset of vague abdominal pain over a few days and was found later to have multiple splenic infarcts. Our patient had a reasonably benign clinical course on conservative management
CASE DESCRIPTION
The patient was a 51-year-old woman with a medical history of primary PV, chronic abdominal aortic occlusion treated with aortobifemoral bypass surgery, left limb ischaemia, and right below-knee amputation. She was on hydroxyurea for the PV. She was referred from a long-term facility after she experienced gradual onset of vague and generalized abdominal pain that progressed over a few days. The pain was associated with food aversion, multiple episodes of nausea and vomiting, and partial response to simple analgesia and pantoprazole.
Upon examination, her heart rate (HR) was 95 beat/min, respiratory rate (RR) was 20, and BP was 128/72 mmHg. Her abdomen was soft, with generalized mild tenderness. Laboratory investigations revealed a white blood cell count of 5×103/µl (normal: 4–10×103/µl), haemoglobin of 10 g/dl (normal: 12–15 g/dl), and a haematocrit of 36% (normal: 36–46%).
An ultrasound of the abdomen was done and showed multiple new splenic lesions (Fig. 1). A computed tomography scan of the abdomen showed the lesions were highly suggestive of splenic infarcts (Fig. 2).
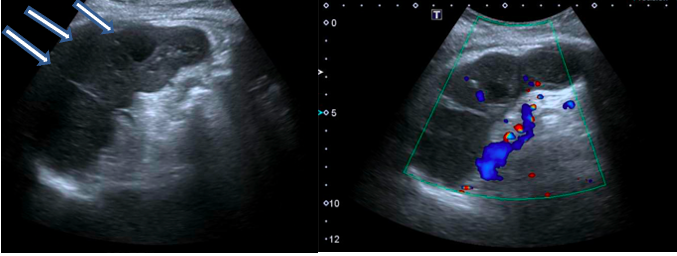
Figure 1. Abdominal ultrasonography (left) showing a heterogeneous echotexture with multiple hypoechoic lesions (arrows) in the spleen with no obvious internal vascularity seen on the Doppler image (right)
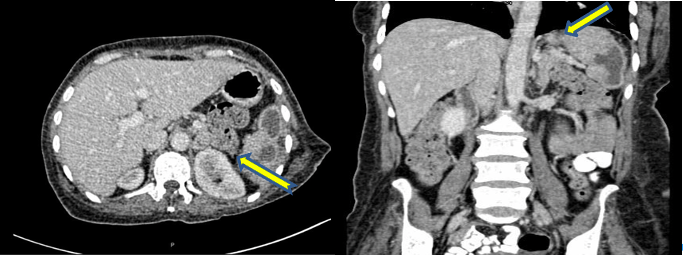
Figure 2. Transverse (left) and coronal views (right) of abdominal CT scans depicting multiple variably sized hypodense non-enhancing lesions within the spleen (arrows), favouring splenic infarction
The patient was managed conservatively. She was started on aspirin, and her hydroxyurea dose was increased. The abdominal pain persisted and eventually localized to the left upper quadrant. The pain management team were involved and provided morphine and tramadol which relieved the patient’s discomfort. She was discharged to a long-term facility and her analgesics were tapered off in 2 weeks. She remained pain free. She was regularly followed by the haematology team who decreased her hydroxyurea dose after a couple of weeks.
DISCUSSION
Splenic infarction results from splenic parenchymal ischaemia that causes tissue necrosis due disruption of arterial blood flow to the spleen [7]. Splenic infarction can manifest as left upper quadrant pain and left shoulder pain (Kehr’s sign). Constitutional symptoms such as fever and chills may also be present. Patients can also develop haemorrhagic shock due to massive sub-scapular bleeding. Splenic infarction has different aetiologies, the most common being a thromboembolic disorder [8]. Infiltrative haematological diseases also induce hypercoagulable states and increase the risk of splenic thrombi and infarction. Autoimmune disease and inherited hypercoagulable states such as protein C and S deficiency also predispose to splenic infarction [8].
PV is a risk factor for splenic infarction and was reported as a rare complication with unknown incidence. PV is a chronic myeloproliferative disease characterized by excessive proliferation of erythrocytes, leukocytes and thrombocytes [8]. It increases bloodviscosity and is associated with arterial or venous thrombosis. Thromboembolic events commonly occur shortly before or at diagnosis and decrease with time, likely due to treatment effects. Age (≥60 years old), a history of thrombosis, and elevated haematocrit and leucocytosis are associated with an increased risk of thrombosis [9]. Splenic infarction is a catastrophic problem [10] and prompt detection and diagnosis are critical [11]. According to the 2016 World Health Organization guidelines, the diagnosis of PV requires the presence of either three major criteria or two major and one minor criteria. The major criteria are: (1) haemoglobin >16.5 g/dl in men and >16 g/dl in women, or haematocrit >49% in men and >48% in women, or red cell mass >25% above the mean standard predicted value; (2) a bone marrow biopsy showing hypercellularity with trilineage growth (panmyelosis); and (3) the presence of JAK2V617F or JAK2 exon 12 mutations. The minor criterion is an erythropoietin level below normal [12].
The complications of PV are classified into four groups: (1) blood clot complications such as deep vein thrombosis, coronary artery clots, hepatic blood clots causing Budd–Chiari syndrome, stroke, and spleen infarction. Blood clot pathogenesis is multifactorial and includes platelet, RBC and WBC anomalies as well as endothelial cell dysfunction [13]; (2) splenomegaly, caused by increased blood viscosity which raises the workload of the spleen; (3) the high number of RBCs which cause gastrointestinal ulcers to develop and bleed; and (4) bone marrow overwork which can lead to diseases such as myelofibrosis, myelodysplastic syndrome and acute leukaemia [4].
Splenic infarction is diagnosed radiologically [14]. It is seen as inconspicuous hypoattenuating areas in unenhanced scans, becomes considerably more distinct after intravenous contrast, and appears as a wedge-shaped, sharply contoured hypodense lesion on CT scanning. Focal infarcts are best demarcated during the early and subacute phases and become isogenous and atrophic in chronic stages. Atypical appearances such as round, heterogeneous or poorly marginalized lesions may give rise to diagnostic controversy or may be mistaken as an abscess, haematoma or tumour [8].
Risk stratification for PV management should be based on age (≤60 versus >60 years) and history of prior thrombosis. Patients ≤60 years old with no history of thrombosis are classified as low risk, and all others as high risk. Phlebotomy is mainly used to treat low-risk PV in order to maintain the haematocrit at <45%, while low-dose aspirin also can be used for high-risk PV. In addition to phlebotomy and aspirin, cytoreductive therapy with hydroxyurea, interferon or busulfan is part of routine management. Patients with PV and thrombotic events should be treated with appropriate doses and intensity of anticoagulation. However, no randomized studies have examined the optimal duration of anticoagulation in a PV patient with a first episode of venous thromboembolism [15].
Our patient was admitted as a case of limb ischaemia but complained of abdominal pain. Abdominal ultrasound showed multiple hypoechoic lesions in the spleen, while CT of the abdomen confirmed multiple variably sized hypodense non-enhancing lesions within the prominent spleen. The haematology team assessed the patient and increased her dose of hydroxyurea. She improved with conservative management without the need for surgical intervention.
CONCLUSION
In our case, splenic infarction was most likely due to a thromboembolic event secondary to PV. In accordance with the literature, we suggested conservative therapy to avoid the need for surgery.