ABSTRACT
Arterial thrombosis and Budd–Chiari syndrome are rare conditions in lung cancer patients. We report the case of a 53-year-old woman who presented with respiratory symptoms, lumbar pain, weight and appetite loss, and an x-ray showing a lung nodule and diffuse micro-opacities. She was diagnosed with lung neoplasia with extensive lung, liver, lymph node and bone metastases. After discharge she was readmitted with a respiratory infection, and as her condition deteriorated, computed tomography was performed and revealed ischaemic areas in the spleen and kidney, and venous thrombosis, related to Budd–Chiari syndrome, with hepatic ischaemia. Despite hypocoagulation, her clinical condition deteriorated and she died soon afterwards.
LEARNING POINTS
- Acute ischaemic arterial events are rare in cancer patients.
- Budd–Chiari syndrome associated with lung cancer is rare.
- Both presentations have a poor prognosis, so early diagnosis and intervention are imperative.
KEYWORDS
Lung cancer, arterial thromboembolism, Budd-Chiari syndrome
CASE DESCRIPTION
A 53-year-old woman was referred to our emergency service due to abnormal chest radiography. She had been asymptomatic until 4 months before this evaluation, when she noticed a non-productive cough that then became productive, with haemoptysis 1 week before admission. She also referred minor dyspnoea, back pain, loss of appetite and weight loss in the previous month, but had no history of fever or night sweats. She presented with a chest x-ray revealing a nodule in the right lung and diffuse reticular and micronodular opacities. She had a background of benign breast cysts and unilateral oophorectomy due to a benign neoplasm at a young age, and was a former smoker with a history of 9 pack-years about 30 years ago. She was only medicated with an oral contraceptive and dietary supplements.
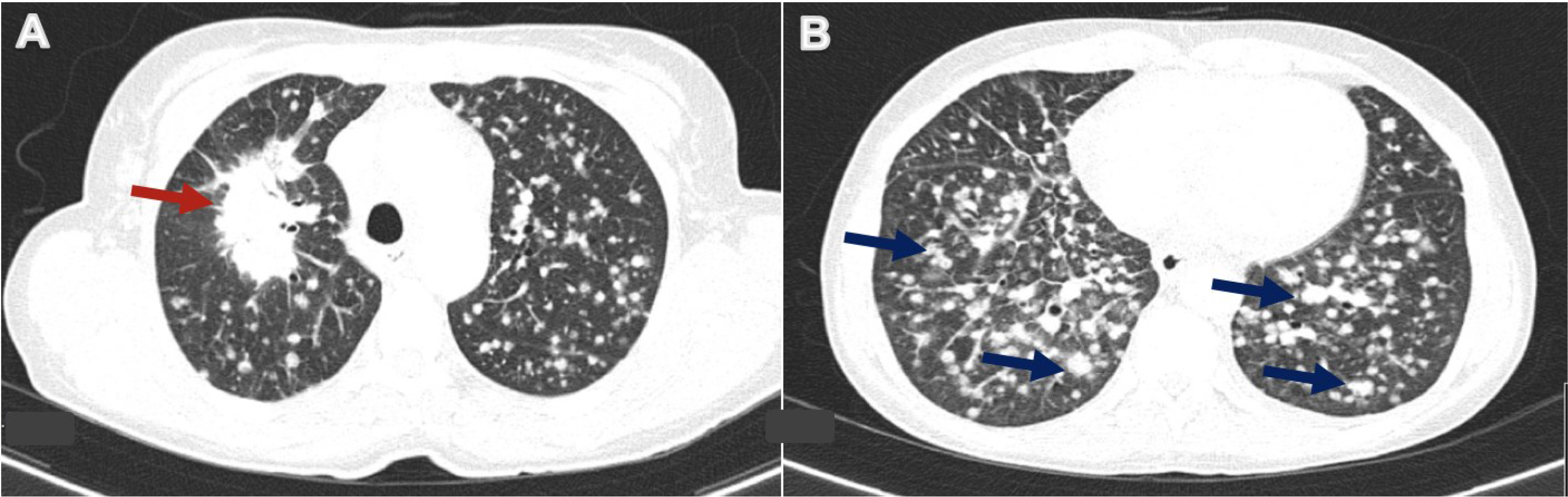
On physical examination, her body temperature was 37.3°C, heart rate was 101 beats per minute, blood pressure was 162/100 mmHg, and oxygen saturation was 98% while breathing ambient air. She was eupnoeic and breathing comfortably. Lung auscultation revealed diffuse crackles, and she exhibited firm cervical lymph nodes on the left. She did not have anaemia, and had a haemoglobin level of 13.3 g/dl (reference range within our centre: 12.0–16.0), a normal white blood cell count of 8.0×103/μl (reference range: 4.8–10.8) and a lymphocyte count of 1.2×103/μl (reference range: 1.0–4.8). Blood electrolytes, liver and renal function were normal, while C-reactive protein was elevated with a value of 58.3 mg/l (reference range: <3.0). Abdominal, pelvic and thoracic computed tomography (CT) revealed a 5 cm spiculated mass in the upper lobe of the right lung, associated with randomly diffuse infracentimetric nodules (Fig. 1A,B), mediastinal lymphadenopathies and suspicious liver lesions. These findings were most consistent with lung malignancy, although concomitant miliary tuberculosis could not be ruled out. The patient was admitted for further study.
Figure 1. Chest computed tomography (CT) revealed a 5 cm spiculated mass in the upper lobe of the right lung (red arrow) (A), associated with randomly diffuse infracentimetric nodules (some examples indicated with blue arrows) (B).
Sputum Ziehl–Neelsen staining, mycobacterium polymerase chain reaction and interferon-gamma release assays were negative, ruling out miliary tuberculosis. Bronchoscopic examination revealed diffuse inflammation of the right bronchus mucosa and obstruction of subsegments of the anterior and posterior right upper lobes. Bronchial aspirate cytology shown the presence of a non-small cell carcinoma and endobronchial biopsy revealed a TTF-1 negative adenocarcinoma with a possible lung origin, although secondary involvement could not be excluded. Gastric and colonic endoscopy, breast, thyroid and gynaecological imaging were all negative for malignancy. Consequently, the patient was observed by Pneumo-Oncology and discharged from hospital. She was awaiting additional investigation, later to be evaluated for further treatment by the Pneumo-Oncology Multidisciplinary Team.
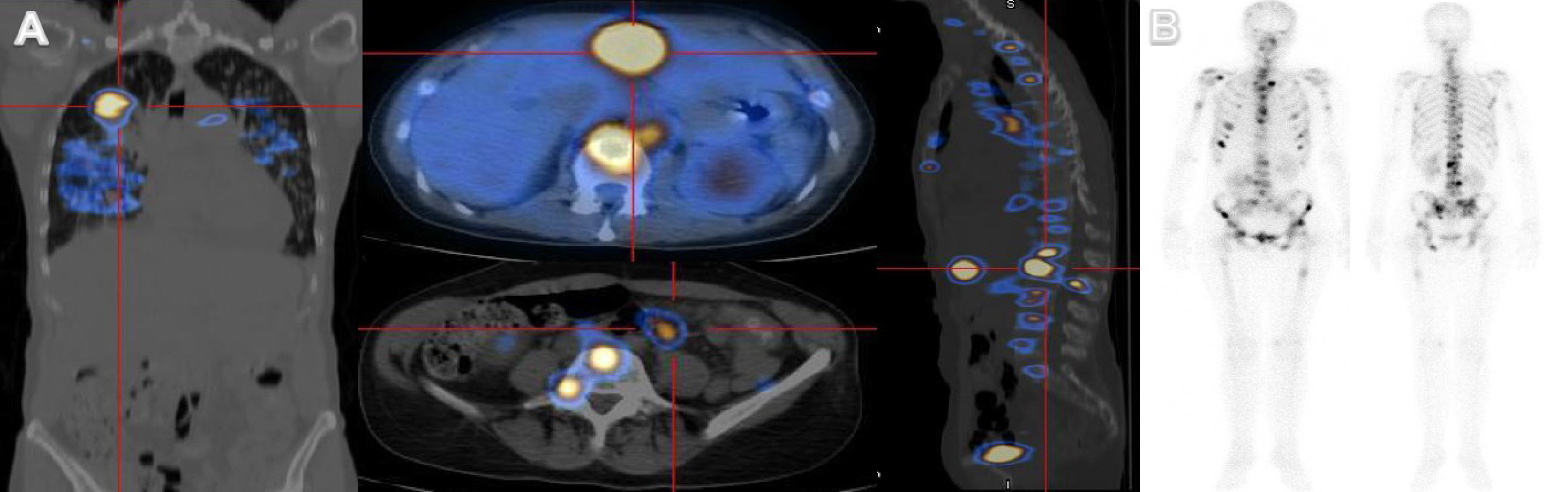
Meanwhile, a positron emission tomography (PET) scan showed a hypercaptation pattern, suggesting primary malignant neoplasia in the right upper lung with bilateral lung, hepatic, bone and lymph node involvement (Fig. 2A). Bone densitometry also revealed multiple secondary bone lesions (Fig. 2B).
Figure 2. Positron emission tomography (PET) scan showing a suspicious malignant lung neoplasm in the right upper lung with bilateral lung, hepatic, bone and lymph node involvement (A). Bone densitometry revealed multiple secondary bone lesions (B).
Diagnosed with stage IVB lung adenocarcinoma, with an Eastern Cooperative Oncology Group (ECOG) performance status of 1, she was proposed for antalgic radiotherapy and palliative chemotherapy with carboplatin plus pemetrexed, as the biopsy did not show any standard mutations that would allow targeted therapy. However, just before chemotherapy initiation, the patient presented with shortness of breath, productive cough, fatigue, loss of appetite and uncontrolled pain, and was again admitted.
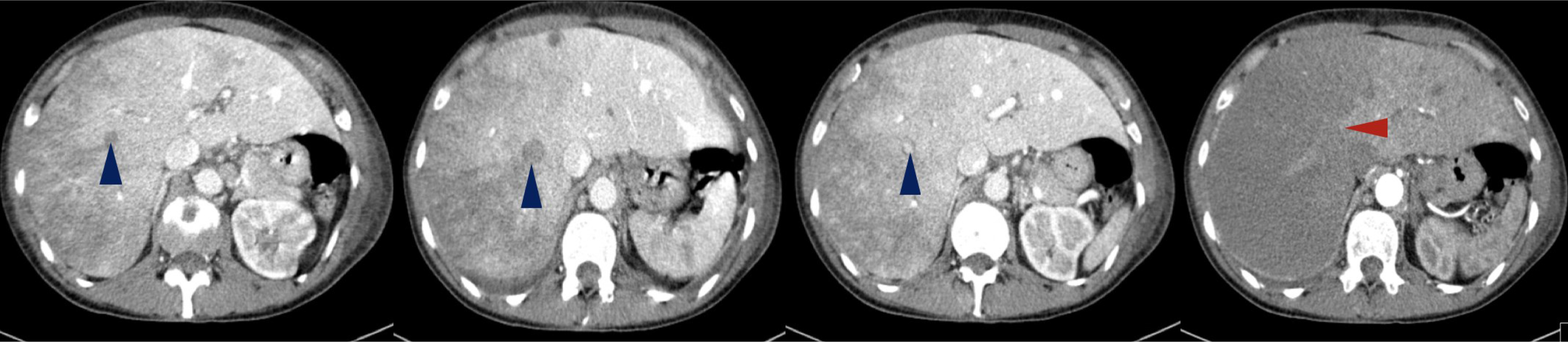
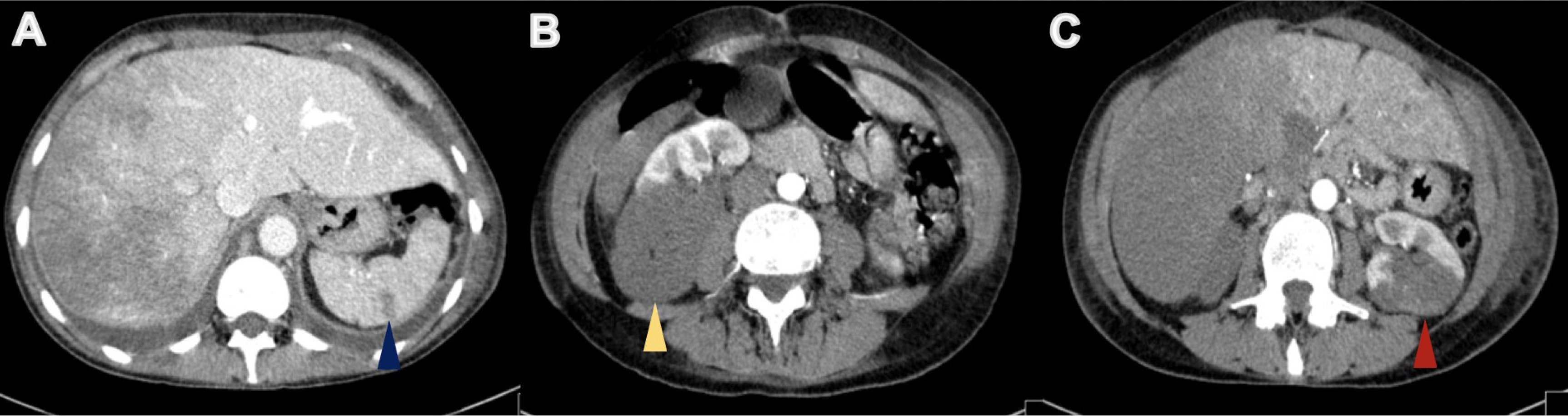
On physical examination, her body temperature was 36.2°C, heart rate was 111 beats per minute, blood pressure was 131/72 mmHg, and oxygen saturation was 84% on ambient air. She was tachypnoeic and uncomfortable, and lung auscultation revealed diffuse crackles. She now presented anaemia with a haemoglobin level of 9.9 g/dl, a white blood cell count of 24.9×103/μl and an elevated C-reactive protein of 106.0 mg/l. Renal function was normal, but she presented hyponatremia of 128 mEq/l (normal range: 135–146) and slightly elevated liver enzymes, with aspartate aminotransferase (AST) of 55 IU/l (normal range: 15–37) and alanine aminotransferase (ALT) of 114 IU/l (normal range: 30–65). Her chest x-ray revealed multiple infiltrates and she was empirically medicated with piperacillin tazobactam and admitted for treatment. During her hospital stay her condition deteriorated and by the third day of admission, she had developed respiratory distress and desaturation. She presented a white blood cell count of 24.2×103/μl, a C-reactive protein of 297.0 mg/l, AST of 4580 IU/l and ALT of 2731 IU/l, but had normal renal function. An abdominal, pelvic and thoracic CT scan was performed (with intravenous contrast administration) and revealed: the known lung mass, now 7 cm in size, plus diffuse secondary lung lesions and carcinomatous lymphangitis, but no lung thromboembolism; defects in filling of the median and right suprahepatic veins (venous thrombosis related to Budd–Chiari syndrome) and marked hypodensity of the right liver lobe, with recent ischaemic phenomena (Fig. 3); spleen hypodense lesions suggestive of recent splenic stroke (Fig. 4A); and considerable areas of stroke in the two upper thirds of the right kidney (Fig. 4B), also visible but smaller in the left kidney (Fig. 4C).
Therapeutic hypocoagulation with enoxaparin was promptly initiated, but she died on the 6th day of hospitalization.
Figure 3. Abdominal CT revealed filling defects in suprahepatic veins with venous thrombosis related to Budd–Chiari syndrome (blue arrowheads). Diffuse heterogeneity of the hepatic parenchyma with hypodensity of the right lobe is due to possible ischaemia (red arrowhead).
Figure 4. Abdominal CT revealed areas of ischaemia in the spleen (blue arrowhead), right kidney (yellow arrowhead) and left kidney (red arrowhead) (A, B and C, respectively)
DISCUSSION
Lung cancer is the main cause of death from malignancy worldwide, accounting for 18% of cancer deaths in 2020 [1]. Thromboembolic events are common in cancer patients and most importantly, they are the second leading cause of death in this population [2]. Acute ischaemic arterial events (AIAE) are rare in cancer patients, with a prevalence of 1.5–3.1% and represent a poor prognosis [2]. The available data on the risk of AIAE in cancer patients are scarce, although some studies have revealed an increased risk with leukaemia, lung, gastric, pancreatic, colon and prostate neoplasia [3, 4]. This risk seems to be increased with the presence of advanced disease, high tumour burden and infection [3, 4], as appeared to be the case in our patient. Other non-cancerous factors such as pulmonary disease, renal disease, cardiovascular risk factors, blood transfusion, chemotherapy and advanced age may also be important risk factors [2, 4]. The aetiology may be thrombotic or embolic, due to the malignancy itself and its treatment, which can promote a hypercoagulable state, peripheral embolization and/or direct tumour compression of the arterial walls. Identifying the exact mechanism may be challenging and the outcome is generally poor [2, 4]. There is a wide spectrum of clinical presentations which include stroke, acute coronary syndrome, and visceral or limb ischaemia [2]. In our case, ischaemia was found in the spleen and kidney, which are rarely affected locations [5, 6]. There are few reports describing similar presentations of arterial thromboembolic phenomena associated with lung cancer [5–7].
Budd–Chiari syndrome is characterized by occlusion of the hepatic vein or inferior vena cava and can present as fulminant, acute, chronic or asymptomatic disease [8]. It may be caused by direct compression of vascular structures, invasion or a malignancy-associated hypercoagulable state [8]. This uncommon condition more frequently occurs with haematological disease, and hepatic and renal carcinoma, and is rarely associated with lung cancer, where it has a poor prognosis [8, 9].
We describe a rare case of simultaneous AIAE and Budd–Chiari syndrome secondary to lung cancer. Although rare, these events may have devastating consequences and early diagnosis and intervention are imperative. Therefore, they should be considered in the differential diagnosis of acutely ill lung cancer patients. Further research is needed in order to develop better therapeutic strategies to improve the survival of these patients.