ABSTRACT
Rosai-Dorfman disease (RDD) is an uncommon lymphoproliferative disorder; RDD with oropharyngeal involvement is extremely rare, especially in adults. A 65-year-old woman with a complaint of progressive dyspnoea since 2016 presented with laryngeal involvement of RDD. A laryngoscopy examination revealed two solid, polypoid masses in the subglottic region, and a laryngeal biopsy concluded chronic inflammation without signs of malignancy. A second biopsy of axillary lymph nodes was performed, supporting the diagnosis of histiocytosis. The patient was treated with corticosteroids and then lost to follow-up. In 2019, she suffered from dyspnoea and a hoarse voice. Laryngoscopy examination showed a polypoid lesion causing airway obstruction at 70% and thickening of the lateral wall of the cavum. Physical examination found left axillary and submandibular adenopathy, and computed tomography revealed thickening of the supraglottic larynx narrowing the laryngeal pathway. Lymphadenectomy with immunohistochemical analysis revealed typical protein positive S-100 histiocytes and emperipolesis. The patient was treated with high doses of corticosteroids for six weeks then these were progressively decreased. The outcome was favourable; the laryngeal lesion disappeared after two weeks of treatment.
LEARNING POINTS
- Rosai-Dorfman disease is a rare cause of lymphadenopathy in adults. Extranodal presentation of the disease is possible mainly in the head and the neck region.
- The diagnosis is based on histological examination with the presence of histiocytes, which are S-100 positive, CD68 positive, and CD1a negative immunohistochemistry.
- The outcome is usually good in asymptomatic forms of the disease with no critical organ involved. The surgical resection is appropriate to the localised symptomatic form of the disease while corticosteroids are indicated in disseminated RDD as a first-line therapy.
- Inspired by our case, rare localisation of Rosai-Dorfman disease (RDD), led to clinical and therapeutic issues. That is why a review of the literature must be undertaken, to share experiences.
KEYWORDS
Rosai Dofmann disease, laryngeal involvement, steroids
INTRODUCTION
Anomalous aortic origin of the right coronary artery (AAORCA) is an uncommon congenital malformation with varying clinical presentations ranging from asymptomatic to sudden cardiac death (SCD)[1]. Those with symptoms or high-risk lesions are usually managed by unroofing or reimplantation, rather than with coronary artery bypass graft (CABG), to avoid late attrition of bypass conduits[2]. Here, we present a case of AAORCA treated with a saphenous graft following intraoperative spasm of the RCA. The literature on the outcomes of those treated with bypass grafting and reimplantation is reviewed.
CASE DESCRIPTION
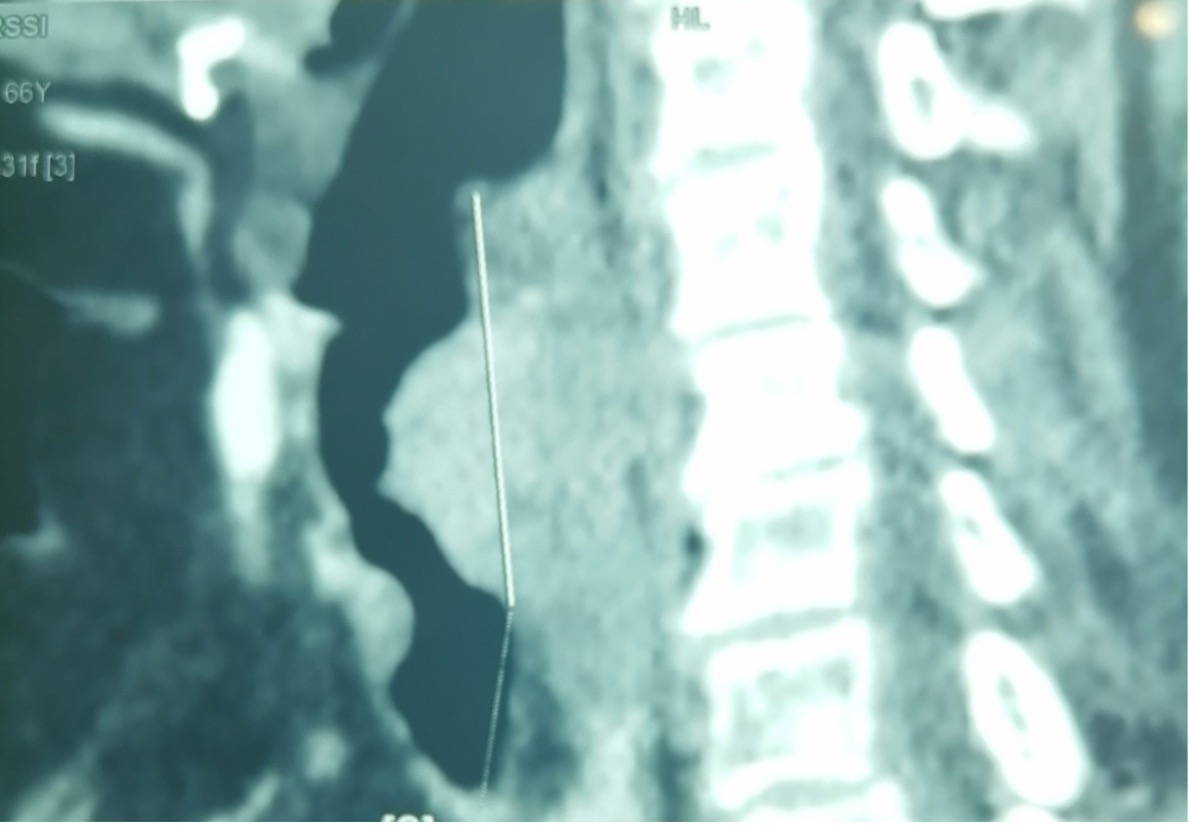
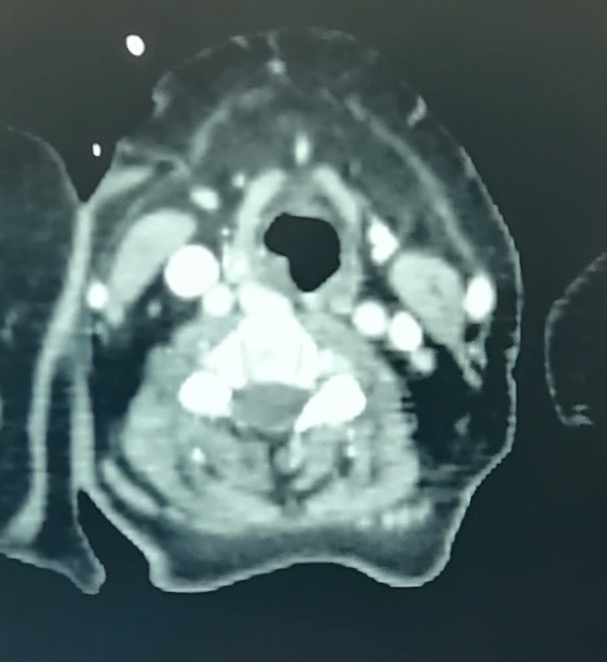
A 65-year-old woman with medical history of hypertension was admitted to our Department of Internal Medicine in December 2019 for progressive dyspnoea. Her medical history started in 2016 when she was complaining of dyspnoea. She was treated by inhaled corticosteroids for bronchitis. Nevertheless, she maintained an expiratory dyspnoea with a hoarse voice. A laryngoscopy examination was made in April 2018, which revealed two solid, polypoid masses in the subglottic region. These nodular lesions had smooth surfaces and were located at 1cm under the right vocal cord and at 1.5cm under the left vocal cord. A laryngeal biopsy concluded chronic inflammation without signs of malignancy. Then, the area was explored by computed tomography of the thorax revealing left axillary adenopathy measuring 4-3 cm in diameter. The biopsy of this adenopathy was performed in September 2018 and supported the diagnosis of histiocytosis. Microscopic examination revealed a preserved architecture of the lymph node with predominant histiocytes, which had an abundant acidophilic cytoplasm, and vesicular nuclei containing numerous lymphocytes within intracytoplasmic vacuoles. The histiocytes were strongly positive with S-100 protein by immunohistochemistry. This finding was concordant with RDD. The patient was treated by corticosteroids for two weeks and then lost to follow-up. In December 2019, she presented to our department with worse inspiratory dyspnoea and a hoarse voice. In the physical examination, she was polypneic at 20 cycles/minute with sus sternal respiratory depression and stridor. We also palpated an axillary lymph node measuring 40-30mm. Pulmonary auscultation was normal; an electrocardiogram found a left branch block and a laryngeal nasofibroscopy examination found bilaterallesion had caused an airway obstruction at 70%. Computed tomography showed circumferential thickening in the subglottic region measuring 13 mm extended over 33 mm, narrowing the laryngeal pathway with compression of the cervical oesophagus (Figs.1 and 2). The biopsy of the axillary lymph node was performed. The histological finding showed that the nodal architecture was preserved with a diffuse infiltrate of histiocytes. The sinusoids contained numerous large histiocytic cells; the majority had smooth nuclear contours and abundant pale cytoplasm. Emperipolesis was seen as intact haematolymphoid cells floating freely in the cytoplasm of histiocytes. Immunohistochemical analysis revealed typical S-100 protein positive. The infiltrate was negative to CD1a and CD207 (unfortunately we did not have histological images since the biopsy was seen in a private laboratory and the histological images could not be provided to us). Corticosteroids were started as an emergency. The patient received high doses of steroids (1 mg/Kg/day). At 15 days of treatment, control laryngoscopy found a resolved tumour and the laryngeal lesion had disappeared. The patient was treated with high doses of corticosteroids for six weeks which was then progressively decreased. The outcome was favourable (the laryngeal lesion had disappeared under a physical examination and in tomography).
Figure 1. Computed tomography, sagittal section, revealing the occlusive lesion of the larynx measuring 3.7 centimetres
Figure 2. Computed tomography, axial section showing the obstructive lesion
DISCUSSION
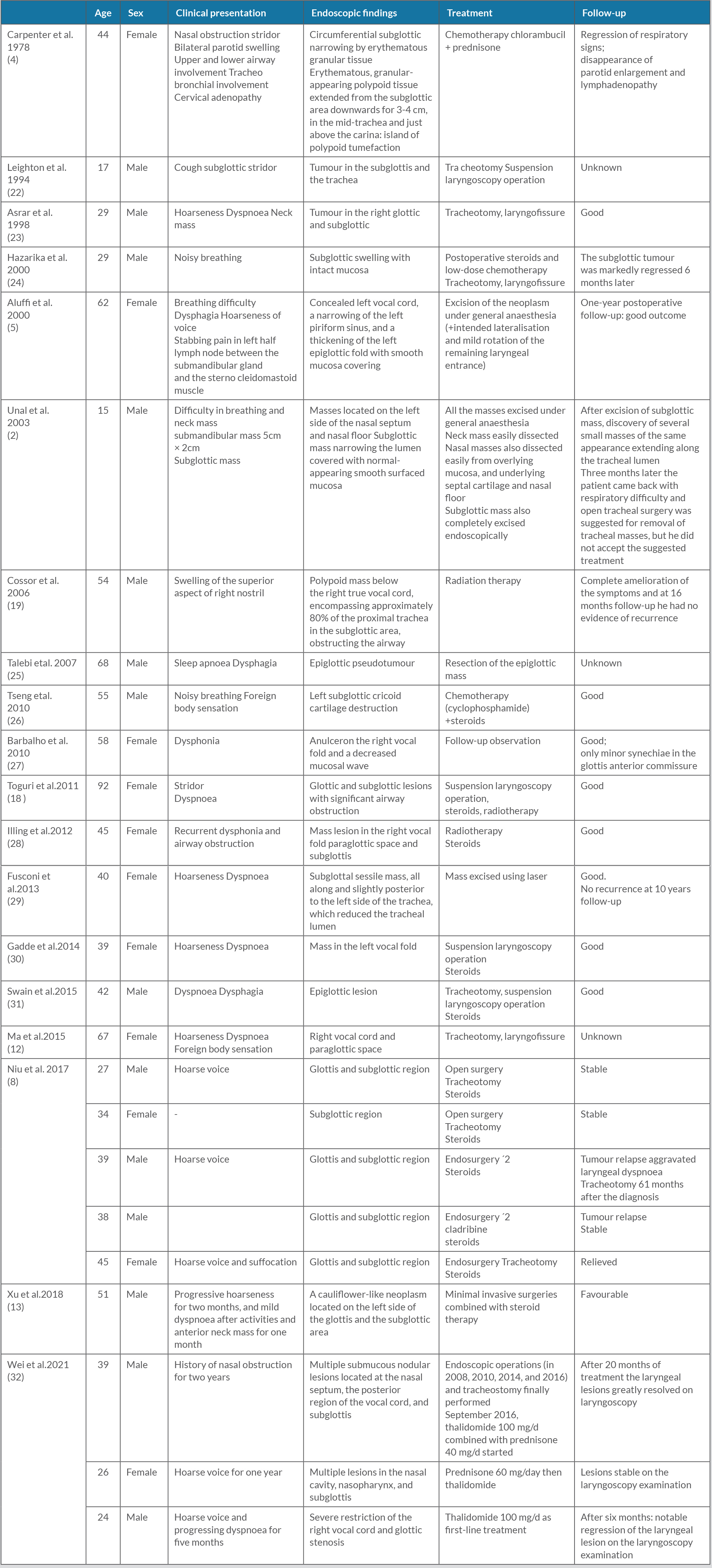
RDD is a non-Langerhans cell histiocytosis characterised by accumulation of activated histiocytes in affected tissues. It occurs in isolation or in association with autoimmune or neoplastic diseases, and includes classic nodal and extranodal disease. It may be sporadic or familial[9,10]. Clinical manifestations in extranodal RDD patients depend on the location of disease. Laryngeal involvement is an uncommon extranodal site rarely affected, according to the literature[8,11], and fewer than 30 such cases reporting laryngeal involvement published[12] (Table 1). This localisation constitutes a diagnosis problem because of the frequency of malignant tumours in this region, which rules out biopsy; this can also often fail to provide a definite diagnosis[13]. In the majority of cases, lesions were unilateral or asymmetric nodular masses similar to our case[8]. Histological examination of extranodal RDD was characterised by the presence of an obvious fibrosis with fewer histiocytes. The immune histochemistry revealed that these cells were S-100 positive, CD68 positive, and CD1a negative; staining with GMS and PAS was negative[8,14,15]. RDD generally had a good prognosis, but laryngeal involvement is a dangerous location because it is life threatening. Dyspnoea due to occlusive laryngeal lesion sometimes indicates surgical intervention as the primary choice[16,17]. Glucocorticoids may be prescribed as the first-line therapeutic option for systemic treatment[8].Radiotherapy could be used in steroid resistant form[18,19], and chemotherapy was also prescribed[13,20]. In a series including 12 patients with laryngeal RDD[13], steroid therapy was used in five patients. Six patients were treated by laryngeal microsurgery under suspension laryngoscopy, three had laryngofissure, and three cases were treated with chemotherapy or radiotherapy, or follow-up observation. The outcome was unknown in three cases and good in the other cases; 20% of patients with extranodal RDD had spontaneous remissions, and 70% of patients require treatment because of disease in a vital organ that was involved[8,21].
CONCLUSION
Rosai Dorfman’s histiocytosis is a benign disease characterised by clinical polymorphism. Its prognosis depends on the seriousness of the locations, particularly oropharyngeal involvement. Symptomatic localised disease is managed with surgical resection or radiation. Symptomatic disease as in the case of our patient is treated with glucocorticoids at high doses as the first-line therapy.