ABSTRACT
Fibrinogen deficiencies are very rare. Qualitative fibrinogen deficiencies (dysfibrinogenaemia and hypodysfibrinogenemia) are functional disorders that can present with both haemorrhagic symptoms and with thrombotic phenomena as unique and paradoxical manifestation. We present the case of a 77-year-old man being investigated for a partially thrombosed abdominal aortic aneurysm as well as an ischaemic stroke 20 years previously. Basic coagulation tests were normal but extended tests revealed a lengthened thrombin time (TT) combined with a significant drop in fibrinogen concentration measured with the Clauss assay and by nephelometry. After secondary fibrinogen deficiencies were ruled out, a heterozygous variant in the FGG gene was detected by next-generation sequencing, and congenital hypodysfibrinogenemia was diagnosed. Acenocumarol was initiated and no new thrombotic or haemorrhagic events had occurred after a year of follow-up.
In almost 25% of cases, thrombotic events may be the only clinical manifestation of functional fibrinogen deficiencies. They are a rare cause of thrombophilia, and are probably underdiagnosed due to normal standard coagulation test results as well as a possible absence of haemorrhagic events. Consequently, a TT test (an initial ‘rule out’ test) should be requested in order to promptly identify these patients. Moreover, discrepancies in derived and Clauss fibrinogen test results should suggest a functional disorder. Finally, new coagulation techniques based on the functional characterization of clot formation, such as ROTEM or thrombin generation assay, could help characterize these entities and suggest new therapeutic approaches.
LEARNING POINTS
- Functional fibrinogen deficiencies can present with thrombotic manifestations only, and are a rare and probably underdiagnosed cause of thrombophilia.
- Thrombin time is a highly sensitive test to rule out other conditions as aPTT and PT results may be within normal ranges, especially in functional deficiencies.
- Discrepancies between derived and Clauss fibrinogen findings, fibrinogen protein measurements and the use of new techniques (ROTEM or thrombin generation) are important for correct approach
KEYWORDS
Fibrinogen deficiency, thrombosis, thrombophilia, congenital hypodysfibrinogenemia, ROTEM
INTRODUCTION
Fibrinogen deficiencies are extremely rare. Qualitative fibrinogen deficiencies (dysfibrinogenemia and hypodysfibrinogenemia) are functional disorders that can present with both haemorrhagic symptoms and thrombotic phenomena. In fact, paradoxical thrombotic events can be the unique manifestation in almost 25% of cases. Normal PT and aPTT findings and sometimes normal derived fibrinogen results along with the unexpected presentation, makes diagnosis difficult. The early identification of this probably underdiagnosed condition is important in order to avoid major thrombotic events. Therefore, this rare deficiency should be considered if a thrombotic event is suspected so that specific thrombophilia studies can be requested.
CASE DESCRIPTION
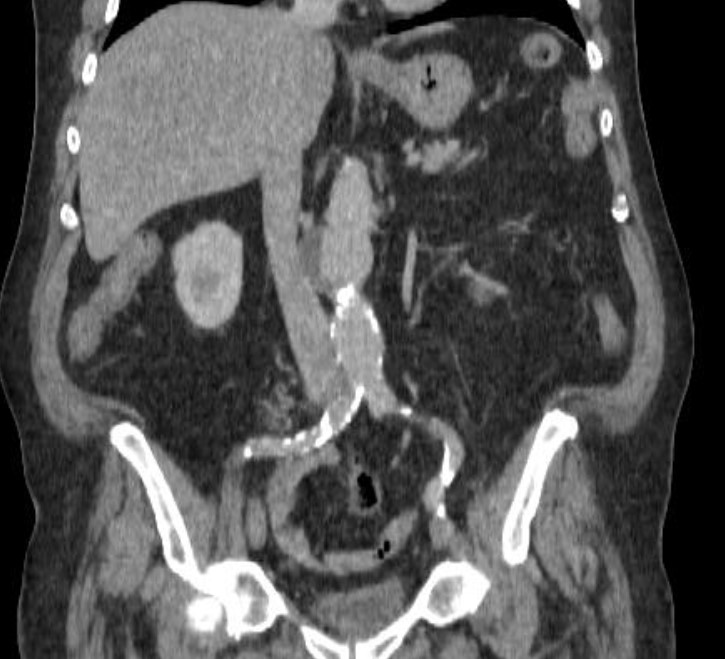
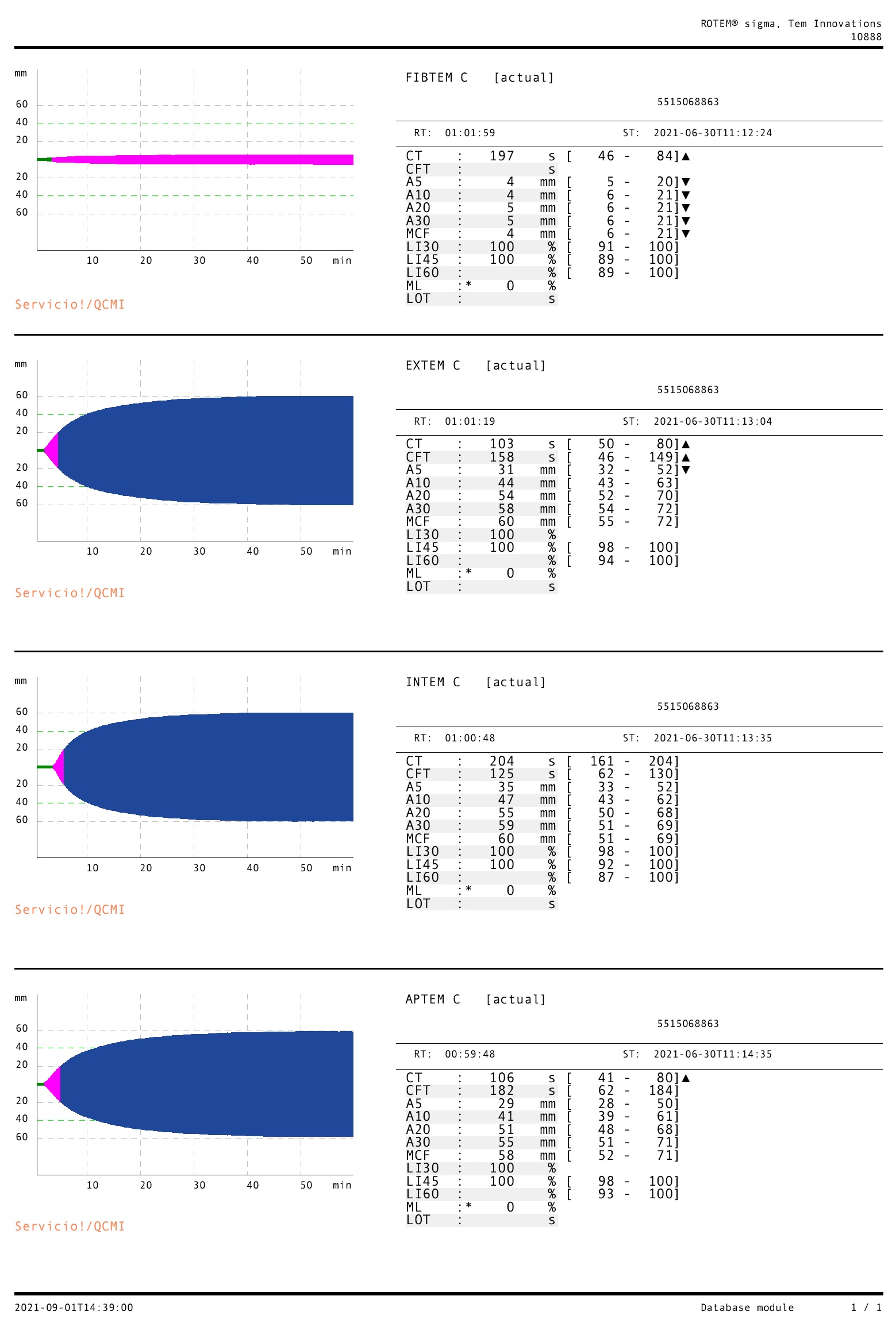
Here we present the case of a 77-year-old male patient referred to our diagnosis unit by the Internal Medicine Department of the Hospital Clínico Universitario de Valladolid for unspecific abdominal pain and a weight loss of 6 kg in the last 3 months. He had a relevant history of ischaemic stroke 20 years ago and was on home medication of 300 mg salicylic acid (SA) daily. Except for mild mesogastric pain on deep palpation, a careful physical examination was normal. Basic laboratory tests only showed slight normocytic and normochromic anaemia along with a normal iron profile. Blood cell counts as well as biochemical parameters (renal and hepatic function, electrolytes and C-reactive protein) were normal. Basic coagulation tests showed normal aPTT, PT and derived fibrinogen values (35.5 s, 11.5 s and 299 mg/dl, respectively). Gastroscopy and colonoscopy did not show any abnormalities. However, computed tomography (CT) revealed a partially thrombosed abdominal aortic aneurysm measuring more than 5 cm in the infrarenal aorta (Fig. 1). A thrombophilia study was requested. Although basic coagulation tests (aPTT, PT and derived fibrinogen) were normal, extended tests revealed a lengthened TT combined with a significant decrease in fibrinogen concentration measured with the Clauss assay and with nephelometry (27.3 s, 56 mg/dl and 1.35 g/l, respectively). Lupus anticoagulant tests (dilute Russell viper venom (dRVV) and silica clotting time (SCT)) were both normal as were blood coagulation factors. We also performed rotational thromboelastometry (ROTEM), an emerging technique which provides real-time and point-of-care information on all stages of clot formation and graphical evidence of fibrinogen deficiency (Fig. 2). No secondary causes of fibrinogen deficiency were found, such as tumour, disseminated intravascular coagulation, hepatic dysfunction or pharmacological therapy, so we considered a possible primary cause (i.e., a congenital fibrinogen deficiency). Study of the FGG, FGA and FGB genes on chromosome 4 by next-generation sequencing (NGS) revealed a heterozygous variant c.1030G>A p. (Asp344Asn) in the FGG gene. Our patient was diagnosed with congenital hypodysfibrinogenemia and anticoagulation therapy with acenocumarol[1] was initiated with international normalized ratio (INR) values within the target range of 2–3. No new thrombotic or haemorrhagic events had occurred after almost a year of follow-up.
Figure 1. Computed tomography (CT) image showing a partially thrombosed abdominal aortic aneurysm.
Figure 2. Rotational thromboelastometry (ROTEM) results. The FIBTEM reagent measures fibrinogen function, which is decreased. The EXTEM, INTEM and APTEM reagents measuring extrinsic, intrinsic and hyperfibrinolysis, respectively, are practically normal, although they do show increased clotting time (CT) which was not evident in conventional tests (PT and aPTT).
DISCUSSION
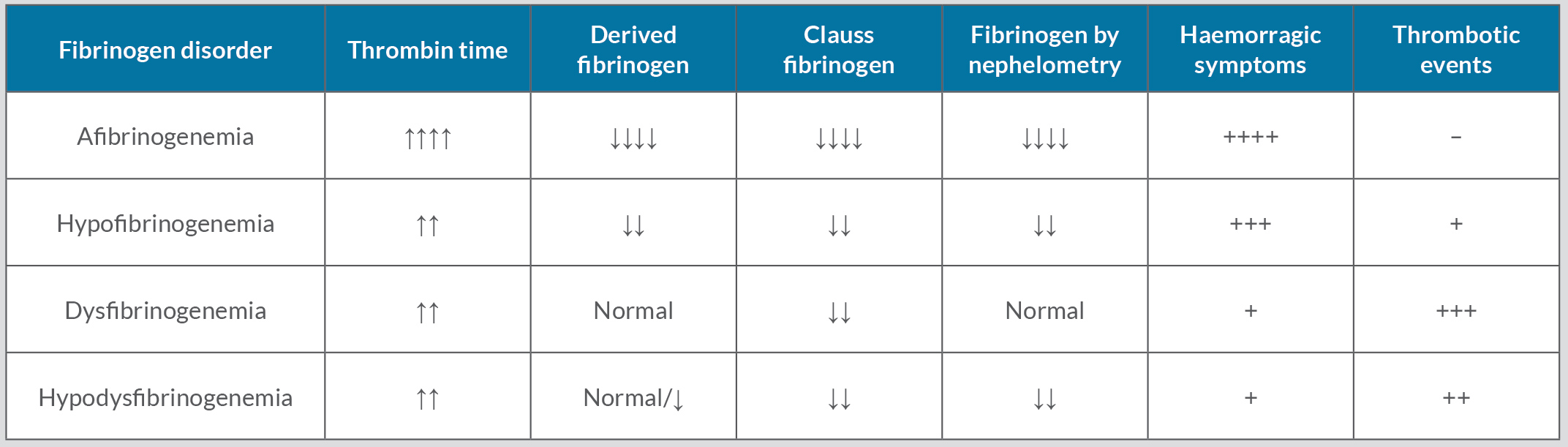
Fibrinogen is involved in secondary haemostasis by polymerizing into fibrin through the action of thrombin[2]. Fibrinogen deficiency is extremely rare, with a prevalence of 1 per million population. The characteristics of the different fibrinogen deficiencies are summarized in Table 1. Deficiencies can be quantitative or qualitative. Quantitative deficiencies are the result of total deficiency (afibrinogenemia) or partial deficiency (hypofibrinogenemia) and present mainly with haemorrhagic symptoms[3,4]. Derived and Clauss fibrinogen values are also decreased. Basic coagulation tests along with significant haemorrhagic symptoms make diagnosis easy, mostly during childhood.
Table 1. Differences between the different types of fibrinogen deficiency.
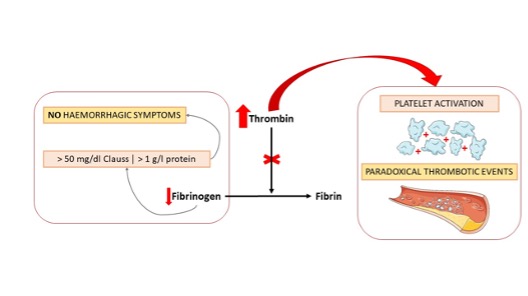
In contrast, qualitative fibrinogen deficiencies (dysfibrinogenemia and hypodysfibrinogenemia) are functional disorders that paradoxically can also be associated with thrombotic phenomena in almost 25% of cases. A further quarter of cases show mild haemorrhagic symptoms while half are asymptomatic. The most important finding is the discrepancy between derived and Clauss fibrinogen values because PT and aPTT findings may be completely normal. On the one hand, haemorrhagic symptoms will be absent if fibrinogen concentrations measured with the Clauss assay or nephelometry exceed the haemostatic thresholds of 50 mg/dl and 1.35 g/l, respectively. On the other hand, unexpected thrombotic events can occur due to an excessive increase in accumulated thrombin (as it is unable to polymerize dysfunctional fibrinogen), which is the main physiological activator of platelets[2–4] (Fig. 3).
Figure 3. Pathophysiology of the clinical manifestations of fibrinogen deficiency.
Our patient had never had haemorrhagic symptoms (Clauss fibrinogen values remained above the haemostatic threshold) but paradoxically, 20 years previously had experienced an ischaemic stroke with no apparent aetiology. In this situation, new coagulation techniques based on functional characterization of clot formation, such as ROTEM or thrombin generation assay, could help diagnosis and treatment[5]. While TT is very sensitive for detecting fibrinogen deficiency and is used as a basic test as to rule it out, ROTEM may provide better discrimination and characterization. It can measure some parameters not detected with quantitative fibrinogen tests (derived and Clauss fibrinogen) such as maximum clot firmness (MCF) and clotting time (CT), which can facilitate appropriate treatment, correct dosage of fibrinogen concentrates and appropriate anticoagulant therapy despite co-existing haemorrhagic and thrombotic symptoms. However, the effectiveness of ROTEM in predicting the clinical phenotypes of fibrinogen deficiencies has yet to be confirmed in large groups of patients. Moreover, we should highlight the fact that functional fibrinogen deficiencies are a rare cause of thrombophilia[2], but are probably underdiagnosed as patients have normal standard coagulation test results and an absence of haemorrhagic events. Consequently, TT should be measured using basic coagulation tests in order to promptly identify such patients.
CONCLUSION
We have described the clinical course and sometimes unexpected thrombotic manifestations of rare fibrinogen disorders, especially functional deficiencies such as dysfibrinogenemia and hypodysfibrinogenemia. They are a rare cause of thrombophilia and are probably underdiagnosed due to normal standard coagulation test results (PT, aPTT and derived fibrinogen) as well a possible absence of haemorrhagic events. Consequently, TT should be measured to rule out unaffected patients and so promptly identify those affected. Discrepancies in derived and Clauss fibrinogen findings should also suggest a functional disorder. However, new coagulation techniques based on functional characterization of clot formation, such as ROTEM or thrombin generation assay, could provide new opportunities for the characterization and treatment of these entities.