ABSTRACT
Drug Reaction with Eosinophilia and Systemic Symptoms (DRESS) is characterised by skin rash together with visceral organ involvement, lymphadenopathy, eosinophilia and atypical lymphocytosis. The syndrome is clinically heterogeneous, making diagnosis challenging. It has an annual incidence of 2 per 100,000 population and a mortality rate of 2–10%. We describe the first case of DRESS induced by certolizumab, a biologic disease-modifying antirheumatic drug (bioDMARD).
LEARNING POINTS
- Drug Reaction with Eosinophilia and Systemic Symptoms (DRESS) is an uncommon and under-reported syndrome.
- Its recognition is critical for treatment, especially in the emergency setting where most patients first present.
- In the case of unexplained fever, lymphadenopathy, cutaneous rash and characteristic laboratory findings (e.g., eosinophilia), after infectious causes have been ruled out, clinicians should always keep DRESS in mind and consider possible recent intake of a triggering drug.
KEYWORDS
Drug Reaction with Eosinophilia and Systemic Symptoms (DRESS), certolizumab, eosinophilia, rheumatoid arthritis, lymphadenopathy
INTRODUCTION
Drug Reaction with Eosinophilia and Systemic Symptoms (DRESS) is a severe adverse drug reaction with a mortality rate of 2–10%, and is estimated to occur in 2 out of 100,000 patients annually [1, 2]. The triggering drug is identified in 80% of DRESS cases, and is commonly antiseizure medication or an antibiotic. We here present the first case of DRESS induced by certolizumab, a biologic disease-modifying antirheumatic drug (bioDMARD). Diagnosis was delayed due to the fact that the syndrome is clinically heterogeneous, making its recognition challenging.
CASE DESCRIPTION
A 29-year-old Latin American woman presented to our Emergency Department with persistent fever, diffuse painful lymphadenopathy (mainly concentrated in the laterocervical and submandibular areas) and a widespread itchy rash on the trunk and limbs, occurring 1 week after the first subcutaneous administration of 400 mg of certolizumab, a tumour necrosis factor (TNF)-alpha inhibitor.
The patient’s clinical history included polycystic ovary syndrome under dietary therapy and moderately active rheumatoid arthritis (DAS28 = 4) currently treated with steroid therapy following previous failed attempts with DMARDs including methotrexate, hydroxychloroquine and salazopyrin. The patient had undergone the usual screening process (i.e., complete blood count, serum creatinine, aminotransferases, evaluation of comorbidities, vaccinations, screening for hepatitis C, hepatitis B, and latent tuberculosis infection) before certolizumab had been initiated.
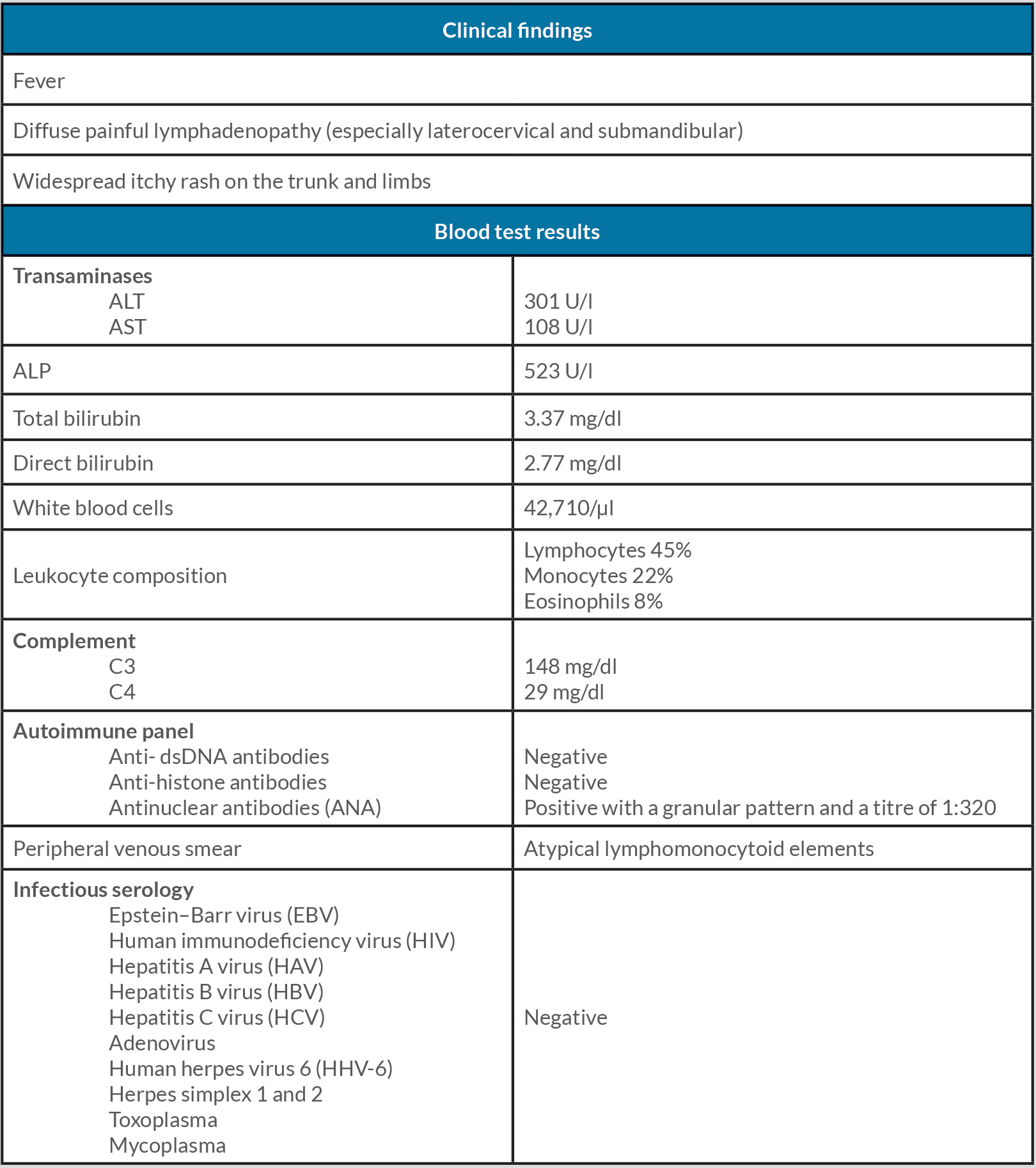
Blood tests showed hepatic impairment characterized by elevated transaminases with increased cholestasis markers (ALT 301 U/l, AST 108 U/l, alkaline phosphatase (ALP) 523 U/l, total bilirubin 3.37 mg/dl, and direct bilirubin 2.77 mg/dl) associated with marked leucocytosis with lymphomonocytosis and eosinophilia (WBC 42,710/µl, with lymphocytes 45%, monocytes 22% and eosinophils 8%) (Table 1).
Further investigation with abdominal ultrasound excluded focal lesions but identified hepatosplenomegaly (16×14 cm). A peripheral venous smear showed atypical lymphomonocytoid elements of variable size and appearance in the absence of blastic forms.
The patient was admitted to our Emergency Medical Unit for observation and care. Blood samples were collected for infectious serology including Epstein–Barr virus (EBV), human immunodeficiency virus (HIV), hepatitis A virus (HAV), hepatitis B virus (HBV), hepatitis C virus (HCV), adenovirus, human herpes virus 6 (HHV-6), herpes simplex 1 and 2, toxoplasma, and mycoplasma IgGs and IgMs, which were all negative. High-dose methylprednisolone (1 mg/kg) and symptomatic therapy with parenteral antihistamine and paracetamol was initiated.
An immunological investigation was carried out. Complement (C3 and C4, with values of 148 mg/dl and 29 mg/dl, respectively) was normal and the autoimmune panel with anti-dsDNA and anti-histone antibodies, was negative, which excluded a vasculitic aetiology and drug-induced lupus. At peak fever, blood cultures were negative, and flow cytometry excluded cell clonality. Antinuclear antibodies (ANA) were positive, attributable to the patient’s active rheumatoid arthritis (granular pattern with a titre of 1:320).
Otolaryngological evaluation with fibrolaryngoscopy carried out to investigate the laterocervical and submandibular adenopathy revealed modest signs of gastroesophageal reflux and hypertrophy of the lymphatic tissue of the base of the tongue. Magnetic resonance imaging (MRI) of the neck confirmed a hyperplasic picture of the lymphatic tissue of Waldeyer's ring, especially the palatine tonsils, and multiple oval lymph nodes increased in size in the laterocervical area without signs of malignancy. During hospitalisation, progressive improvement was seen in eosinophilic leucocytosis (WBC 20,630/µl, with lymphocytes 50%, monocytes 6% and eosinophils 18%) and hepatotoxicity, with gradual normalization of transaminases and decreased cholestasis markers (AST 29 U/l, ALT 46 U/l, ALP 136 U/l and gamma glutamyl transpeptidase (GGT) 360 U/l). Rheumatology confirmed ongoing steroid therapy and suggested a tapering regimen without reintroducing any DMARDs, given the likelihood of possible DRESS induced by certolizumab. After the patient achieved clinical stability (apyrexia, regression of rash and amelioration of lymphadenopathies), she was discharged on oral steroids. Subsequent follow-up visits confirmed clinical remission with no autoimmune sequelae.
DISCUSSION
DRESS is a rare severe adverse drug reaction characterized by an extensive skin rash, with visceral organ involvement, lymphadenopathy, eosinophilia and atypical lymphocytosis [3]. Clinical presentation can be heterogeneous with latency between drug initiation and disease onset of 2–8 weeks, and frequent reactivation of latent viral infection (e.g., HHV-6) [4]. The triggering drug is identified in 80% of DRESS cases (with a possible dose-dependent correlation), drug causality is unclear in 18% of cases, and no drug exposure is present in 2% of cases[4].
Antiseizure medication (carbamazepine, phenytoin and lamotrigine), allopurinol, sulfonamide-containing antibacterials, minocycline and vancomycin are the most common drug triggers [5].
DRESS is considered a T-cell-mediated hypersensitivity reaction. Although its exact pathogenesis is not fully understood, a pathogenetic mechanism involving a drug-specific immune response with human Herpesviridae reactivation followed by an antiviral immune response has been hypothesized as reactivation of HHV-6, HHV-7, EBV or CMV is seen in up to 75% of patients with DRESS [6].
Clinical presentation includes a prodromal phase characterized by non-specific symptoms (fever, lymphadenopathy) with an overt phase characterized by systemic involvement: skin manifestations are the most obvious and start as a maculopapular rash progressing to coalescing erythema distributed over the trunk and extremities, involving >50% of the body surface area [7]. Pruritus may be an accompanying symptom. Mucosal involvement is mild and seen in up to 50% of patients, allowing diagnostic discrimination from Stevens–Johnson syndrome and toxic epidermal necrolysis. Systemic symptoms include fever, lymphadenopathy, haematological abnormalities (eosinophilia >700/µl (95% of cases), leucocytosis (95%), lymphocytosis (25%) and monocytosis (69%)), atypical lymphocytes (35%) and laboratory abnormalities related to visceral involvement. Involvement of at least one internal organ occurs in 90% of patients, often preceding the development of cutaneous rash [8]. Liver damage is the most common visceral manifestation (80%) [9].
The clinical course of DRESS is variable. The average time to recovery is 7 weeks, with 20% of patients having a prolonged course lasting more than 3 months, especially when there is severe liver involvement and atypical lymphocytosis (as occurred in our patient) [10].
A skin biopsy for histopathology should be performed for differential diagnosis with other cutaneous diseases (i.e., lymphomas such as Sézary syndrome and cutaneous lupus erythematosus) with DRESS showing heterogeneous and non-specific characteristics [11]. Due to the heterogeneity of its clinical presentation, DRESS is frequently misdiagnosed and often identified retrospectively.
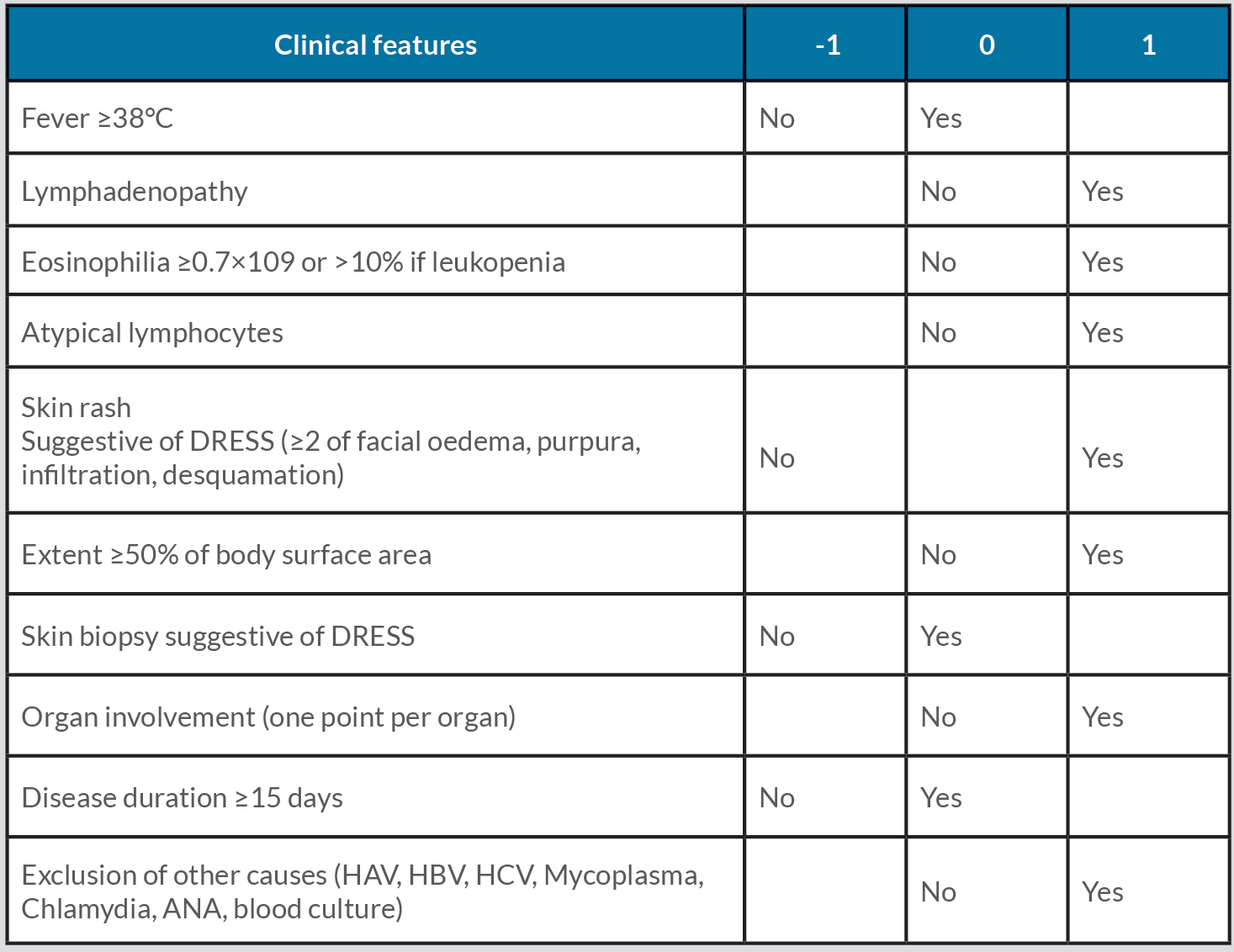
The Registry of Severe Cutaneous Adverse Reactions (RegiSCAR) scoring system is commonly used to confirm or exclude DRESS and enables retrospective validation of suspect cases. The criteria include fever (>38°C axillary), enlarged lymph nodes in at least two different body areas, eosinophilia, atypical lymphocytes, skin involvement (skin biopsy) and organ involvement (at least two-fold elevation of liver enzymes). A value between 1 and 2 is assigned to each criterion with a cumulative score ranging from 4 to 9. The diagnosis is excluded if the score is <2, possible if 2–3, probable if 4–5, and definite if ≥6 [11] (Table 2).
Table 2. Scoring system for the diagnosis of Drug Reaction with Eosinophilia and Systemic Symptoms (DRESS) syndrome
Leucocytosis with eosinophilia was present, atypical lymphocytes were seen on peripheral blood smear, and no infectious causes were identified, in agreement with the literature [8]. There was also liver damage, which is common in DRESS syndrome [9]. The RegiSCAR scoring system provided a total score of 6, which confirmed the diagnosis.
To our knowledge we have described the first case of certolizumab-induced DRESS syndrome, although a few cases caused by other bioDMARDs, such as tocilizumab, have been reported in the literature [14]; bioDMARDs may act as a trigger in genetically predisposed patients. On the other hand, Leman et al. described a case of lithium-induced DRESS effectively treated with infliximab, which is an anti-TNF monoclonal antibody [15]. However, in our opinion, given that the pathogenesis in that case was not fully elucidated, cure was achieved through the discontinuation of lithium. It is difficult to determine how much bioDMARD withdrawal affects clinical remission, because while anti-TNF has an immunomodulating role, allowing steroid tapering in pathologies such as psoriasis, hidradenitis suppurativa, pyoderma gangrenosum and Behcet’s syndrome, the individual variability of patients must also be considered.
CONCLUSION
We here report a rare case of DRESS caused by certolizumab, a drug that has not previously been associated with this syndrome, which is more often due to antibiotics and antiseizure medication. Moreover, the most common side effects of certolizumab (seen in 1 out of 10 subjects) are bacterial infections, hepatitis, leukopenia, eosinophilia, rash and fever, with pruritus rarely present at the same time. Early diagnosis and identification of the causative agent is essential for prompt treatment of DRESS syndrome. However, evidence-based guidelines for diagnosis and best clinical management are lacking due to the great heterogeneity of clinical manifestations. Randomized clinical trials are required to support prompt diagnosis and effective therapy [16].