ABSTRACT
Introduction: Several immune-mediated side effects have been reported with COVID-19 vaccines, including myocarditis.
Case description: A 27-year-old woman with a past medical history of mild COVID-19, developed adult-onset Still’s disease (AOSD) with salmon-pink flagellate erythema, polyarthritis, a sore throat, myocarditis and haemophagocytic lymphohistiocytosis after receiving two doses of the BNT162b2 vaccine (Pfizer®, BioNTech®). Despite the initial efficacy of high-dose pulses of methylprednisolone, inflammatory markers rose as soon as de-escalation of corticosteroids was attempted, warranting initiation of biologics targeting the interleukin (IL)-1/6 axis, which allowed sustained remission of the disease despite withdrawal of corticosteroids.
Discussion: To our knowledge, this is the first case of AOSD with both haemophagocytic lymphohistiocytosis and cardiac magnetic resonance imaging-proven myocarditis triggered by COVID-19 vaccination, successfully treated with steroids and biologics targeting the IL-1/IL-6 axis. The pathophysiological process by which COVID-19 vaccination can lead to AOSD is still unknown, although it has been reported that the spike protein may act as a pathogen-associated molecular pattern and thus induce an overproduction of pro-inflammatory cytokines of the innate immune system (e.g., IL-1, IL-6 or IL-18).
Conclusion: Targeting the IL-1/6 axis is effective for the treatment of severe steroid-refractory BNT162b2 vaccine-induced adult-onset Still’s disease. At a population level, the favourable benefit/risk ratio of COVID-19 vaccination remains indisputable.
LEARNING POINTS
- Adult-onset Still’s disease can be triggered by the BNT162b2 COVID-19 vaccine.
- Biologics targeting the interleukin-1/6 axis should be considered early in the course of vaccine-induced adult-onset Still’s disease, especially when steroids do not effectively curb the disease.
KEYWORDS
Adult-onset Still’s disease, myocarditis, COVID-19, vaccines, biological therapies
CASE DESCRIPTION
Since the beginning of the COVID-19 vaccine campaign established to curb the pandemic, several immune-mediated side effects have been reported, including myocarditis [1]. Here, we report a case of severe steroid-refractory adult-onset Still’s disease (AOSD) with associated myocarditis and features of haemophagocytic lymphohistiocytosis (HLH) triggered by BNT162b2 vaccination, and the subsequent efficacy of interleukin (IL)-1/6 axis blockade.
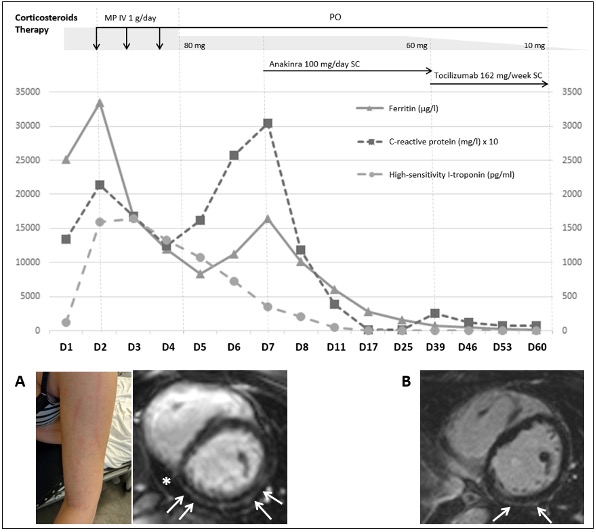
A 27-year-old woman with a past medical history of mild COVID-19 in March 2021, received two doses of the BNT162b2 vaccine (Pfizer®, BioNTech®) in September 2021 and in January 2022. The day after she received the second dose, she developed high-grade fever (40°C), salmon-pink flagellate erythema on the trunk and on the proximal limbs (Fig. 1A), polyarthritis and a sore throat. Despite treatment with paracetamol, symptoms worsened, and the patient was finally referred to our centre a week later. At admission, laboratory findings showed neutrophilia (17×109/l), high inflammatory markers (C-reactive protein 214 mg/l, ferritin >33,511 µg/l, fibrinogen 2.5 g/l), elevated high-sensitivity I-troponin (1600 ng/l, reference value <15 ng/l) and brain natriuretic peptide (181 ng/l), while the glycosylated ferritin value was low (10%). Calculation of the HScore yielded a 82% probability of reactive HLH [2]. Reverse transcriptase polymerase chain reaction testing for COVID-19 was negative twice, and an extensive work-up for infectious, auto-immune and neoplastic causes was negative. As the patient met both the Yamaguchi and Fautrel classification criteria [3, 4], the diagnosis of AOSD was retained. Trans-thoracic echocardiography and coronary computed tomography angiography were unremarkable, while cardiac magnetic resonance imaging (CMR) showed sub-epicardial late gadolinium enhancement (LGE) of the inferior and lateral walls consistent with myocarditis (Fig. 1A). The patient’s condition initially improved with high-dose pulses of methylprednisolone (1 g/day for 3 days), but treatment with subcutaneous anakinra (100 mg/day) was initiated 5 days later when inflammatory markers rose as soon as de-escalation of corticosteroids was attempted. The patient was finally discharged on day 10. Subsequently, despite clear clinical efficacy, anakinra was discontinued because of injection-site reactions and tocilizumab (weekly subcutaneous injections of 162 mg) was started. After 6 weeks of follow-up, despite rapid tapering of corticosteroids, the patient’s favourable course was maintained. Moreover, follow-up CMR 60 days after hospital discharge showed a clear reduction of LGE (Fig. 1B).
Figure 1. Time course and therapeutic management of BNT162b2 vaccine-induced adult-onset Still’s disease in a 27-year-old woman. (A) Typical salmon-pink flagellate erythema on proximal limbs and a short-tau inversion recovery CMR short-axis view demonstrating sub-epicardial LGE (arrows) in the inferior and lateral walls consistent with myocarditis (arrows) along with pericardial effusion (asterisk). (B) Clear reduction in LGE (arrows) on a 60-day follow-up CMR.
CMR, cardiac magnetic resonance imaging; IV, intravenous; LGE, late gadolinium enhancement; MP, methyl prednisolone; PO, per os; SC, subcutaneous
DISCUSSION
AOSD is a multisystemic inflammatory disorder of unknown aetiology mostly affecting young adults, in particular women, that may be triggered by numerous causes, for example viral infections or vaccines [5]. To date, reports of AOSD (including with myocarditis or HLH) triggered by COVID-19 vaccination (whether mRNA or adenovirus vector vaccines) are scarce (n <20) [6–9]. To the best of our knowledge, this is the first case of severe AOSD with both HLH and CMR-proven myocarditis that was successfully treated by steroids and IL-1/IL-6 blocking biologics. Although the association between AOSD and COVID-19 vaccination could be coincidental, the strong temporal correlation between both events in our patient and previous reports of both immune-mediated events (AOSD and myocarditis) following the administration of the BNT162b2 vaccine or other types of vaccines (including the flu and hepatitis vaccines) [1, 5, 8] makes the probability of a vaccine-triggered event very likely in our view. The underlying pathophysiological process by which COVID-19 vaccination can lead to AOSD is still unknown, but the SARS-COV2 spike protein seems to play a key role since cases of AOSD have been reported with both types (i.e., mRNA and adenovirus vector) of vaccines [6–8]. Hence, this spike protein may act as a pathogen-associated molecular pattern and thus induce an overproduction of pro-inflammatory cytokines of the innate immune system (e.g., IL-1, IL-6 or IL-18) owing to Toll-like receptor activation [10], in particular TLR2 or TLR41. Of note, such cytokines are also involved in both the pathophysiology of AOSD itself and the COVID-19-induced cytokine storm [11].
First-line treatment of AOSD relies on systemic corticosteroids. In steroid-refractory cases, depending on the clinical presentation, second-line drugs mostly consist of disease-modifying antirheumatic drugs, TNFα inhibitors (chronic arthritis), and/or anti-IL-1/6-targeted biological therapy (systemic AOSD) [5]. Overall, severe COVID-19 vaccine-related adverse events are rare [1]. In the present case, despite a severe initial presentation, the patient’s course was excellent owing to the prompt initiation of IL-1/IL-6 blocking drugs. At a population level, the favourable benefit/risk ratio of COVID-19 vaccination remains clear.