ABSTRACT
We present the case of a patient with a history of symptomatic hypoglycaemic episodes and a negative 72-hour fasting test with histological confirmation of insulinoma. A literature review of hyperinsulinaemic hypoglycaemia with a negative fasting test was performed.
LEARNING POINTS
- The 72-hour fasting test is the gold standard for insulinoma diagnosis.
- Few cases of insulinoma with a negative fasting test have been reported.
- New strategies for insulinoma diagnosis are being investigated.
KEYWORDS
Insulinoma, hyperinsulinaemia, fasting
INTRODUCTION
Insulinoma is a usually benign neuroendocrine tumour which causes sporadic endogenous hypoglycaemia and is associated with genetic syndromes in up to 10% of patients. The gold standard for insulinoma diagnosis is the 72-hour fasting test. Different imaging studies may help locate the tumour, which is essential as surgical management is the only curative strategy [1].
CASE DESCRIPTION
A 65-year-old male patient, with a medical history of grade I obesity and obstructive sleep apnoea, consulted after two episodes of postprandial loss of consciousness associated with spontaneous hypoglycaemia. A capillary glucose test after the first episode showed 54 mg/dl; no capillary glucose test was registered after the second episode. The patient required assistance on both episodes, and recovery was observed after an oral glucose load.
Thereafter, the patient was evaluated in the outpatient clinic. HbA1c was 4.5% and a pancreatic mass was observed on abdominal computed tomography (CT). The patient was referred for inpatient evaluation as an insulin-producing tumour was suspected.
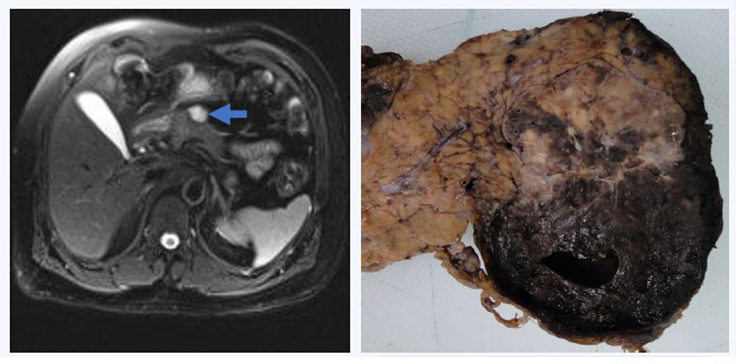
A 72-hour fasting test was performed under strict surveillance. The patient was allowed to drink calorie-free beverages or water. Capillary glucose samples were collected every 2 hours and venous blood glucose samples every 6 hours until the plasma glucose concentration fell to a limit close to 60 mg/dl. If this value is reached and the patient remains asymptomatic per protocol, the sampling frequency should be increased to every 2 hours and if glucose levels fall below 60 mg/dl, insulin, C-peptide, proinsulin, acid beta-hydroxybutyrate and sulfonylurea should be measured. The 72 hours were completed with no evidence of hypoglycaemia (capillary glucose test nadir: 64 mg/dl), thus concluding in a negative test result. The chromogranin A result was 11.7 ng/ml (reference value: 0–100 ng/ml). Contrast abdominal magnetic resonance imaging was performed, which showed a bilobate solid and cystic lesion, partially exophytic, on the pancreatic neck, measuring 47×38 mm, iso and hypointense on T1 and hyperintense on T2 (Fig. 1).
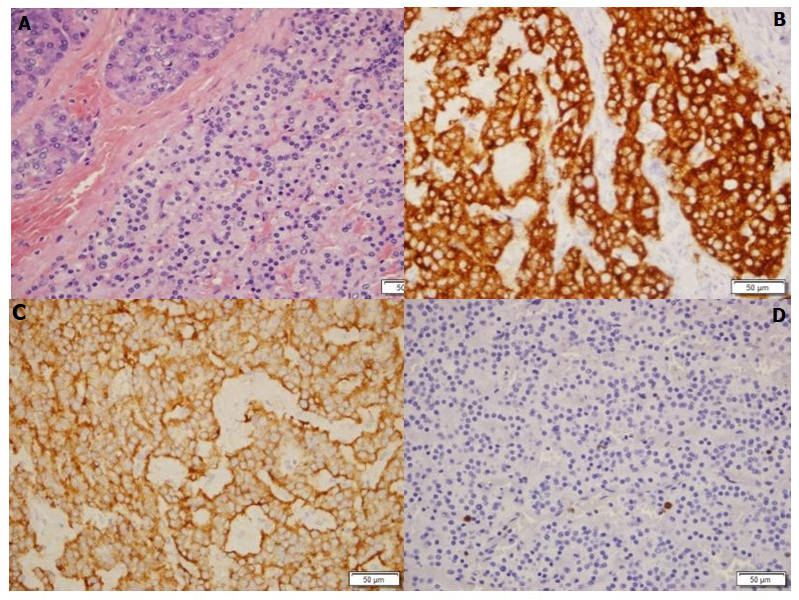
The patient underwent subtotal (90%) pancreatectomy, splenectomy and regional lymphadenectomy using a hybrid technique. Hyperglycaemia was observed on post-surgery surveillance, which was managed with insulin therapy. Histology revealed a well-differentiated neuroendocrine tumour with a cystic and solid pattern (KI67 of 1%), consistent with a grade ⅓ insulinoma (Fig. 2).
Figure 1. Left: Mixed mass (solid and cystic) in the pancreatic neck measuring 47×38 mm and hyperintense on T2 (arrow). Right: Lesion in the neck of the pancreas measuring 58×32 mm, solid and cystic, with extensive bleeding and clots
Figure 2. Histological results. (A) Haematoxylin: left side: normal pancreas, right side: nest of tumour cells. (B) Synaptophysin and (C) insulin (400×): positive in neuroendocrine cells. (D) Ki67 (400×): proliferation index of 1%
DISCUSSION
Insulinoma is a rare neuroendocrine tumour with an incidence of 4 cases per million persons/year [2]. Inappropriately elevated insulin levels cause hypoglycaemia [1], which may be associated with autonomous nervous system symptoms such as palpitations, diaphoresis, weakness and anxiety, or neuroglycopenic symptoms such as blurry vision, confusion and loss of consciousness [3]. Hypoglycaemia is usually observed during fasting states. However, this may vary as some series have found that 73% of patients present symptoms exclusively during fasting, 6% postprandial, and 21% in both states [4].
The 72-hour fasting test is considered the gold standard for hypoglycaemia evaluation, with a sensitivity of 88.9% and a specificity of 100% for insulinoma diagnosis [5]. The test objective is to demonstrate a relationship between symptoms, hypoglycaemia, and inappropriate insulin elevation when hypoglycaemia is observed [6]. Some series have reported a positivity rate of 93% after fasting for 48 hours, and 99% at 72 hours [7].
Despite a high pretest probability, there are few case reports of histology-confirmed insulinoma with a negative 72-hour fasting test [7–10]. In the present case, a negative fasting test was observed in a patient with histology-confirmed insulinoma. This phenomenon could be related to the preservation of normal beta cell properties in tumour cells, such as the capacity to down-regulate insulin secretion in response to low serum glucose levels [8]. Hence, a normal fasting test does not exclude the diagnosis, and must be interpreted according to clinical presentation. Therefore, for cases with high clinical suspicion, the fasting test could be repeated, or additional tests could be performed such as investigation of serial β-hydroxybutyrate levels or glucagon administration (neither of which are available in Colombia) [9, 11, 12].
Additionally, some outpatient tests have been suggested, such as the 5-hour glucose tolerance test, with diverse indexes described such as 5-hour insulin/glucose and fasting C-peptide/glucose levels (specificity 73.08%, sensitivity 82.67%) [13].
Continuous glucose monitoring has also been proposed as a diagnostic test, as it increases the detection of hypoglycaemic episodes, especially asymptomatic episodes which occur at night. This could reduce the time required for investigation and treatment response evaluation. The use of different tools such as the glucose variation coefficient (VC), M value, low blood glucose index (LBGI) and continuous overall net glycaemic action (CONGA) could help to differentiate insulinoma-related hypoglycaemia from other causes of hypoglycaemia[14, 15].
As this is a relatively rare entity and most studies have been performed on small groups, larger studies are required to further investigate new outpatient diagnostic strategies which could reduce inpatient costs.