ABSTRACT
Introduction: Subclavian artery stenosis (SAS) is a manifestation of peripheral artery disease (PAD). Presentation varies, ranging from arm claudication and muscle fatigue to symptoms which reflect vertebrobasilar hypoperfusion, among which are syncope, ataxia and dysphagia. Although rare, severe bilateral SAS can exist and present as refractory hypotension. We describe a case of bilateral SAS masquerading as circulatory shock, or rather ‘pseudoshock’.
Case Description: A 59-year-old female patient presented to the emergency department with complaints of dark stools. She was anaemic and hypotensive and therefore suspected to have an acute gastrointestinal bleed (GIB) with resultant haemorrhagic shock. Her hypotension was unresponsive to fluid resuscitation and blood transfusions. Bilateral upper extremity radial artery catheters confirmed low blood pressures. After her blood pressure failed to improve despite the addition of several vasopressors, a femoral artery catheter (FAC) was placed, which revealed significant hypertension discordant with the hypotension measured by the radial artery catheters. Review of CT angiography of the upper extremities revealed the presence of bilateral SAS which was deemed to be the aetiology of the falsely low blood pressure.
Discussion: SAS should be suspected in patients with lower extremity PAD or a blood pressure (BP) differential of 15 mmHg or more between arms. When bilateral subclavian arteries are stenosed, this difference in BP may be concealed, making lower extremity BP measurements, as seen in non-invasive tests such as ankle brachial index (ABI) tests or through more invasive procedures such as FAC placement, critically important.
Conclusion: Bilateral SAS may present as pseudo-hypotension. In cases of refractory shock of unclear aetiology, especially in patients with known PAD, a high index of suspicion is warranted for ‘pseudoshock’ secondary to severe vascular stenosis. Comparison of upper and lower extremity BP via invasive arterial catheters or non-invasive ABI tests can aid in the diagnosis of bilateral SAS.
LEARNING POINTS
- Bilateral subclavian artery stenosis (SAS) may present as pseudo-hypotension and shock of unclear aetiology.
- In patients with underlying peripheral arterial disease, pseudoshock should be considered in the differential diagnosis.
- Comparison of upper and lower extremity blood pressure via invasive arterial catheters or the non-invasive ankle brachial index (ABI) test has diagnostic value for bilateral SAS.
- Pseudoshock is managed via secondary prevention with antiplatelets and statins for asymptomatic patients, and revascularization for symptomatic patients.
KEYWORDS
Bilateral subclavian artery stenosis, vertebrobasilar insufficiency, shock, pseudoshock, peripheral vascular disease, vasopressors
INTRODUCTION
Subclavian artery stenosis (SAS) is an uncommon condition, and reported to have a prevalence of 1.9% in the community population and 7.1% in clinical studies in patients with vascular disease [1]. The true incidence of bilateral SAS is unknown due to its extreme rarity. Based on our literature review, refractory hypotension has been reported in less than five cases of bilateral SAS. SAS should be suspected in patients with a faint pulse in the upper extremities and a systolic blood pressure (BP) difference of more than 15 mmHg between the right and left upper extremities [1].
Rarely, bilateral SAS has presented as pseudo-hypotension. In a case described by Hirata et al, bilateral SAS was misdiagnosed as cardiogenic shock [1–3]. In the setting of severe vascular stenosis and refractory shock of unknown origin, the index of clinical suspicion for pseudoshock should be raised. The use of invasive arterial catheters or non-invasive ankle brachial index (ABI) tests to compare upper and lower extremity BP can help diagnose bilateral SAS. If done promptly, either approach can prevent the possible unwanted sequelae of excessive and unnecessary vasopressor use. We present a case of bilateral SAS which initially masqueraded as haemorrhagic shock, and where a missed diagnosis conferred significant morbidity.
CASE DESCRIPTION
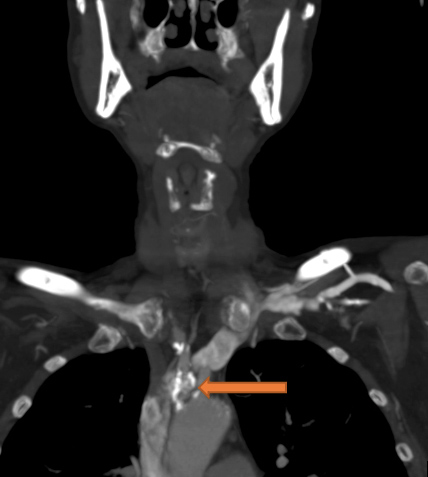
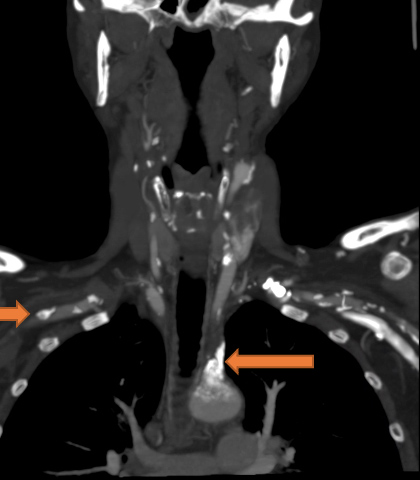
A 59-year-old woman with a history of cirrhosis and severe peripheral vascular disease (PVD) presented to the Emergency Department with dark stools and dizziness. She was found to have acute anaemia (Hgb of 6.2 g/dl), hypotension with BP 64/55 mmHg, and tachycardia of 105 bpm. Due to an inadequate response to 3.5 litres of normal saline resuscitation and 6 units of blood transfusions, intravenous (IV) norepinephrine was instituted. The patient was admitted to the intensive care unit for suspected hypovolemic/haemorrhagic shock secondary to a possible gastrointestinal bleed (GIB). A right radial artery catheter (RAC) was placed for invasive BP monitoring which confirmed persistent low blood pressure. Vasopressor requirements quickly escalated to maximum doses of IV norepinephrine, vasopressin, epinephrine and phenylephrine. A left RAC was placed, which confirmed the accuracy of BP readings obtained from the right radial artery. Nasogastric lavage was unrevealing for blood; haematochezia and melena were not observed. Thorough review of the patient’s past medical history revealed severe peripheral artery disease (PAD) with several recanalization procedures involving the popliteal arteries, superficial femoral artery, external iliac artery, common iliac artery, bilateral carotid and femoral endarterectomies. Review of her prior computed tomography angiography (CTA) of the neck revealed very high-grade stenosis/occlusion of the proximal left subclavian artery, as well as extensive calcification with moderate high-grade stenosis of the proximal innominate/brachiocephalic artery and right subclavian arteries (Figs. 1 and 2). These imaging findings in the setting of persistent need for high-dose vasopressors despite normal lactic acid levels and the absence of a source of shock, raised strong suspicion for bilateral SAS. A femoral artery catheter was placed 4 days into her admission revealing a blood pressure of 174/71 mmHg compared with the RAC BP reading of 64/50 mmHg. The patient was subsequently weaned off vasopressors within 24 hours with BP monitoring based on lower extremity pressures
Figure 1. CT angiogram of the neck shows extensive calcifications and narrowing at the proximal innominate artery
Figure 2. CT angiogram of the neck shows calcification and stenosis at the left subclavian and right subclavian artery
It was thus determined that the patient was not truly in shock, but rather had ‘pseudoshock’, a diagnosis supported by artificially low upper extremity BP readings in the setting of severe bilateral SAS. Unfortunately, the patient had an extended hospital stay complicated by bilateral middle cerebral artery (MCA) and right posterior cerebral artery (PCA) territory infarcts, ventilator-associated pneumonia and delirium.
DISCUSSION
The left subclavian artery stems directly from the aortic arch, while the right subclavian artery originates from the innominate/brachiocephalic trunk. Together they supply blood to the upper extremities and much of the head and neck [4]. The vertebral artery is the first vessel to branch off from the subclavian artery and supplies the upper spinal cord, brainstem, cerebellum and posterior part of the brain [4].
SAS is a form of upper extremity PAD which should be suspected when exam findings reveal a BP differential of 15 mmHg or more in the arms [5, 6], especially in patients with known lower PAD. Atherosclerosis has been identified as the most frequent cause, followed by fibromuscular dysplasia, compression syndrome and Takayasu arteritis [2, 7]. The pathophysiology of atherosclerotic SAS has been described as a process of cell adhesion: inflammatory cells attaching to the arterial wall, with arterial wall remodelling and lipid accumulation resulting in calcification. This process is accelerated in patients with hypertension, hyperlipidaemia and diabetes [3].
Common symptoms include upper extremity claudication, muscle weakness, and manifestations of vertebrobasilar insufficiency such as syncope, dizziness, diplopia, tinnitus and/or hearing loss [8]. Significant SAS is typically considered greater than 50% stenosis in the subclavian arteries [6]. Clinical exam findings include diminished upper extremity pulses, supraclavicular bruits, finger ulcerations and necrosis (particularly in those with longstanding disease) [3].
Subclavian steal syndrome occurs when there is significant unilateral stenosis of the subclavian artery proximal to the origin of the vertebral artery, leading to a decreased pressure gradient in the ipsilateral vertebral artery and an increased pressure gradient in the contralateral non-occluded vertebral artery [3]. The difference in this pressure gradient leads to a reversal of flow through the ipsilateral vertebral artery, and blood is shunted from the contralateral vertebral artery, away from the brain, to supply the ipsilateral upper extremity [9]. Subclavian steal syndrome can be a sequela of severe SAS which may present with vertebrobasilar insufficiency; however, it does not occur in bilateral SAS.
When bilateral subclavian arteries are stenosed, the difference in upper extremity BPs may be concealed, making comparison to lower extremity BP measurements, as seen in tests such as ABIs, critically important. Though the ABI test is not validated in longitudinal studies, it has been utilized to diagnose bilateral SAS in previously reported cases [2]. If there is suspicion for SAS but comparison of bilateral upper extremity BP measurements is unrevealing, an ABI showing higher-than-normal values (ABI >1.3) can prompt the provider to investigate for bilateral SAS, and consider more conclusive testing. An ABI value greater than 1.3 can also be seen in non-compressible vessels [10], so this alone should not be used as the sole diagnostic test. Other non-invasive testing that can be used to aid diagnosis includes arterial duplex ultrasound, CTA and magnetic resonance angiography (MRA). Using arterial duplex ultrasound, a high velocity flow can indicate a narrowed vessel and the direction of blood flow can be determined [11]. Images from CTA or MRA will reveal any narrowing, tears, dilations, aneurysms, or other damage to the subclavian arteries [11]. A more invasive angiogram can be done, which includes introducing a catheter into the artery and injecting dye to visualize the path of blood flow, which will then reveal the location and severity of occlusion [11].
Management of SAS in asymptomatic patients relies on secondary prevention with antiplatelet therapy and statins, while revascularization is preferred in symptomatic patients [12]. Options for revascularization include endovascular treatment with balloon angioplasty and stenting. This approach involves inserting a catheter with a balloon which is then inflated to open up the artery, followed by stent placement to keep the artery open [11]. Surgical revascularization options include transposition of subclavian to carotid artery, carotid–subclavian bypass using a synthetic graft, or subclavian–axillary bypass if carotid artery utilization is not feasible [13].
Our patient was referred to vascular surgery, with plans to proceed with surgical revascularization once she recovered from her inpatient medical issues.
CONCLUSION
Pseudoshock should be investigated as a potential cause of an unexplained refractory hypotension especially in the setting of PAD. Simple non- or minimally invasive tests such as measuring the ABI or placement of arterial catheters in both upper and lower extremities can aid in the establishment of an accurate diagnosis in such cases. Prompt identification and management of pseudoshock may prevent the inappropriate and unnecessary use of vasopressor medications, thereby avoiding the adverse effects of uncontrolled hypertension and the complications often associated with prolonged hospitalization.