ABSTRACT
Right atrial masses are rare and diagnosis can be difficult unless histopathological specimens are obtained. In addition, the clinical course is not well documented, thereby making diagnosis and management challenging. The mass can be associated with haemodynamic instability with the potential to cause obstructive shock and embolism. We present the case of a young woman with untreated chronic myelogenous leukaemia with a massive haemodynamically significant right atrial mass. The usefulness of multimodality imaging and a multidisciplinary approach for diagnosing and treating this condition is highlighted.
LEARNING POINTS
- Right atrial mass is rare and can lead to pulmonary embolism and haemodynamic instability.
- As chronic myelogenous leukaemia is associated with an increased risk of thromboembolism, thrombus should be considered in the differential diagnosis of intracardiac masses.
- Multimodality imaging is indicated to guide diagnosis and appropriate management; in case of diagnostic uncertainty, histopathology may be needed to obtain a definitive diagnosis.
KEYWORDS
Right atrial mass, chronic myelogenous leukaemia, histopathology
CASE DESCRIPTION
A 33-year-old woman with BCR-ABL1-positive chronic myelogenous leukaemia (CML) presented with worsening dyspnoea associated with pleuritic chest pain and subjective fever for several days. She denied palpitations, leg swelling, light-headedness, syncope, blurry vision or weakness. Of note, she had given birth to her third child 2 months before admission. She had been treated with interferon since the 14th week of her pregnancy. There was no family history of cancer or heart disease. She denied tobacco, alcohol or illicit drug use.
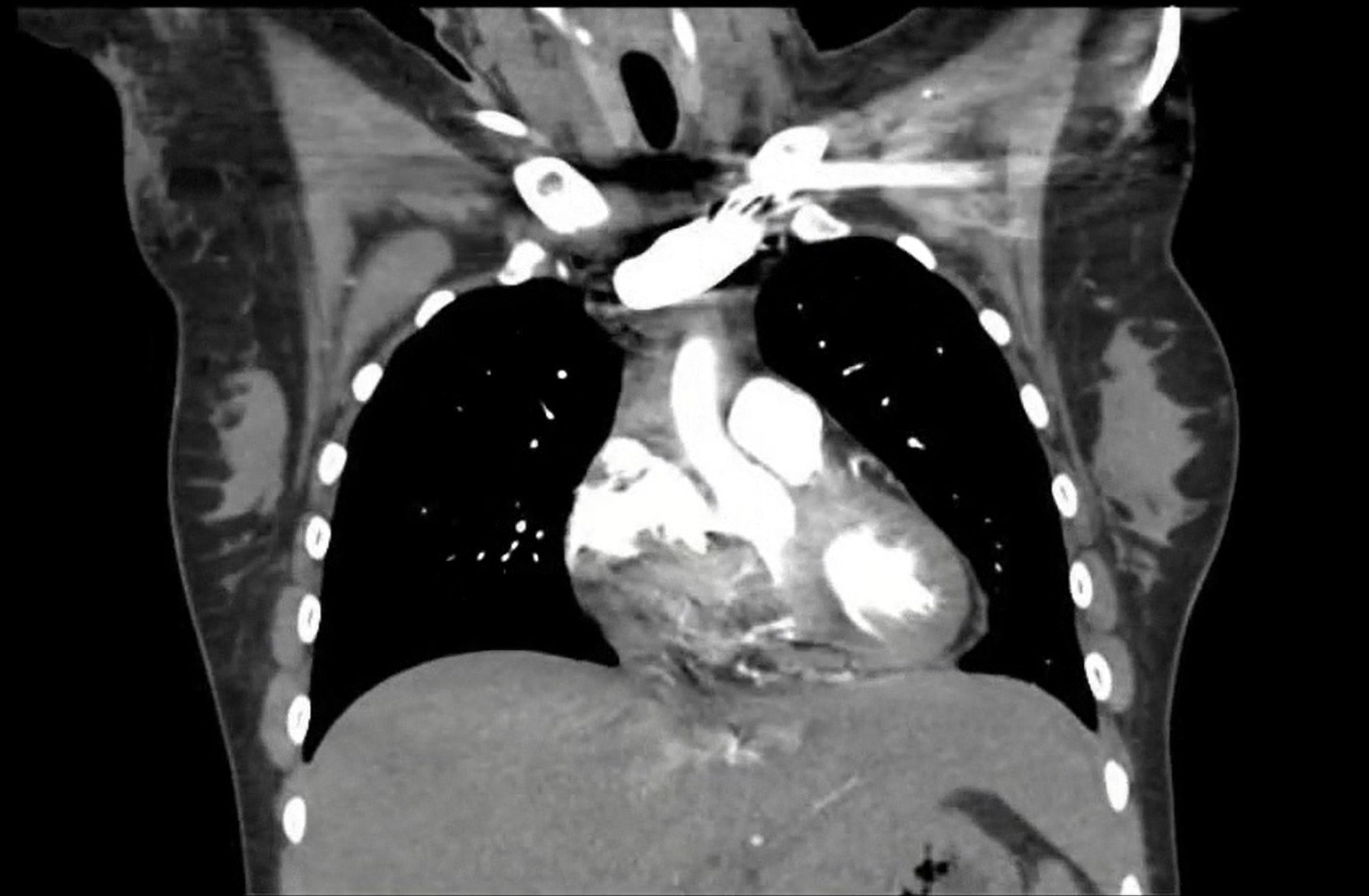
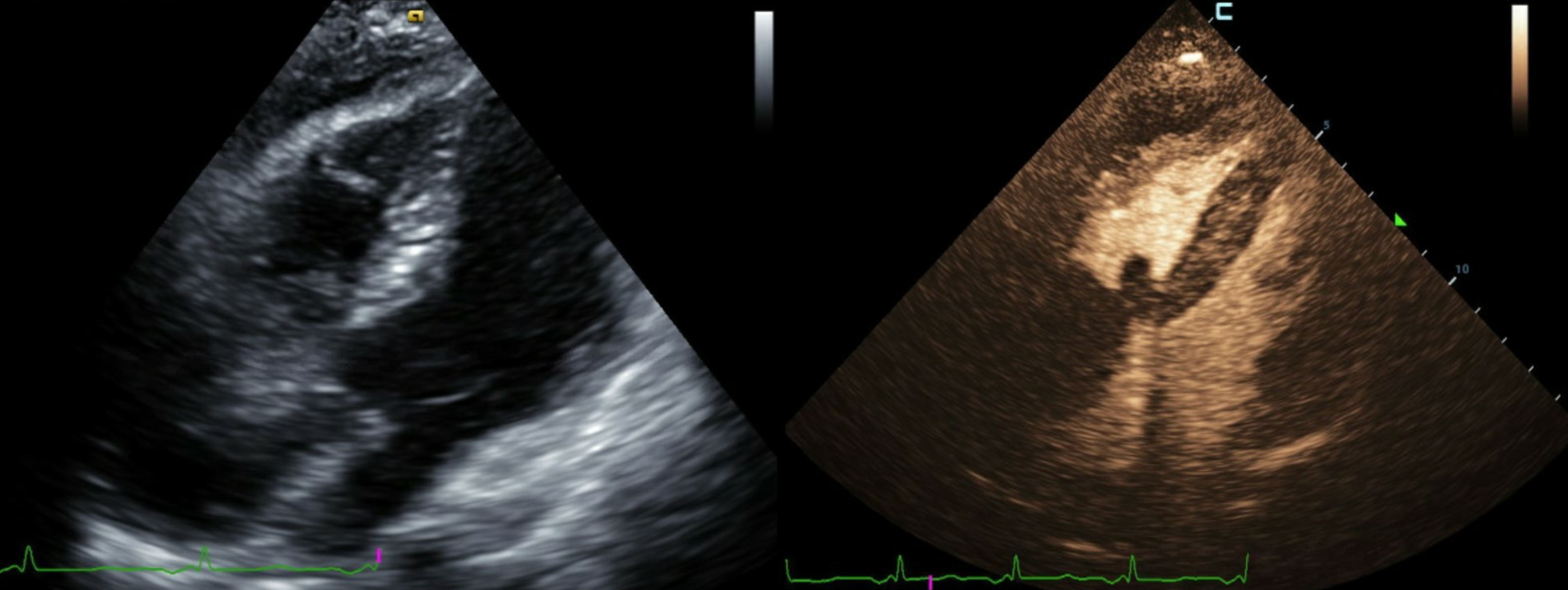
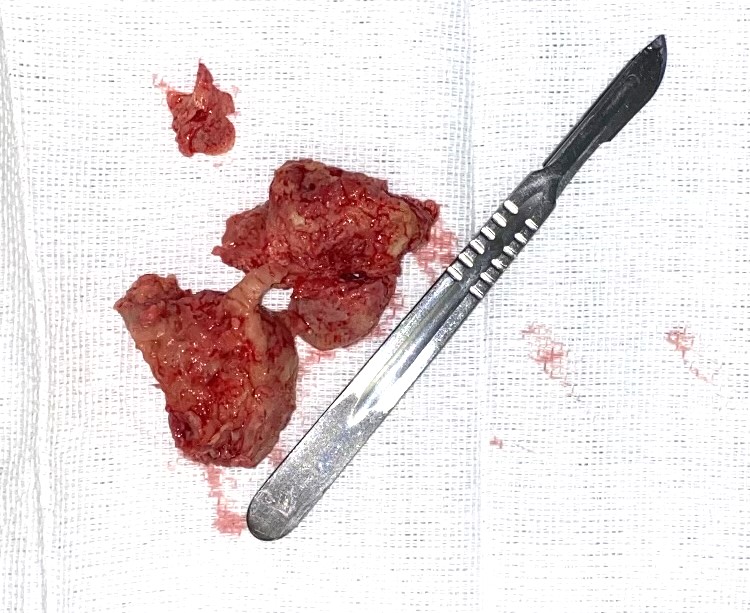
The patient had right-sided heart failure with hypotension which responded to fluids. Laboratory results showed leucocytosis (75×103/mm3), mild anaemia, thrombocytosis, transaminitis (ALT 833 IU/l, AST 1203 IU/l), elevated BUN (4.9 mg/dl) and creatinine (1.7 mg/dl), and lactic acidosis (5.9 mmol/l). Computed tomography (CT) of the heart showed a 6 cm lobulated mass within the contrast pool extending from the right atrium into the proximal right ventricle (Fig. 1) and bilateral subsegmental pulmonary emboli. Transthoracic echocardiography also revealed the mass, which almost obstructed the tricuspid valve opening, and a small pericardial effusion (Fig. 2). A decision to proceed with surgical excision of the mass was made after multidisciplinary discussion between the cardiologist, advanced imaging cardiologist, oncologist and cardiothoracic surgeon. A fist-sized mass was resected from the right atrium (Fig. 3). The mass was firmly attached to, but did not invade, the inferior wall of the right atrium.
It almost completely occluded the tricuspid valve ostium and the coronary sinus. Pathological findings revealed extensive fibrin and focal necrosis consistent with an old blood clot. No malignant cells were identified. Postoperative recovery was complicated with bilateral sterile exudative pleural effusion which was treated accordingly.
Figure 1. Computed tomography demonstrating a large 6 cm lobulated heterogeneous mass in the right atrium extending into the right ventricle
Figure 2. Transthoracic echocardiography with contrast demonstrating a large right atrial mass prolapsing into the right ventricle associated with right-sided chamber dilation and a small pericardial effusion without signs of tamponade
Figure 3. Resected large bilobed mass with histopathological evidence of extensive fibrin and necrosis
DISCUSSION
Intracardiac masses are rare and can be classified as neoplastic or non-neoplastic [1]. The most common triggers are thrombi in the setting of atrial fibrillation or left ventricular systolic dysfunction, and vegetations in the setting of endocarditis [2]. Malignancy is a rare cause of intracardiac masses. The prevalence of primary cardiac tumours is less than 0.03% with atrial myxomas being the most common. Metastatic disease from malignant melanoma, breast and lung cancer is 20–40-fold more frequently encountered than primary tumours [3].
Lipomatous hypertrophy of the interatrial septum is an incidental finding in 1–8% of the general population and exhibits a characteristic dumbbell-shaped homogeneous mass involving the interatrial septum with sparing of the fossa ovalis on cardiac CT [4].
Intracardiac thrombi may occur in any chamber and, on cardiac CT, appear as hypodense, low-attenuation filling defects in a contrast pool[4]. Predisposing factors include hypercoagulable states including autoimmune diseases, pregnancy and certain malignancies [2]. Right atrial thrombi are extremely uncommon and, without histopathological evaluation, obtaining a definite diagnosis can be difficult [5]. A 5×6 cm right atrial mass in a 72-year-old man with end-stage renal disease and fever, in whom myxoma was initially suspected, was found to be an infected thrombus [5].
Thromboembolism is a well-recognized complication of cancer [6]. Data from meta-analyses of more than 160,000 patients with leukaemia suggest that the overall incidence of thromboembolism is approximately 5%, with CML conferring the greatest risk [6]. The exact incidence of intracardiac thrombus in the CML population remains unknown. Our literature review revealed a single case report of a 45-year-old patient with CML who developed left ventricular thrombus attached to the mitral valve apparatus despite having no other cardiac risk factors [7]. In addition to CML, an additional contributing factor in our patient was her postpartum state, which is a period associated with the greatest risk of thromboembolism in pregnant women [8].
Recent advances in non-invasive imaging modalities have been helpful in managing a variety of cardiovascular pathologies. Echocardiography is the first-line method for evaluating cardiac masses due to its widespread availability and cost-effectiveness [1]. If malignancy is suspected, better mass characterization and evaluation of metastasis can be obtained by cardiac CT and/or magnetic resonance imaging (MRI). Cardiac CT, owing to its high spatial and contrast resolution, is particularly useful in assessing calcification, fatty masses, and intrathoracic and vascular structures [1]. Of note, the presumed diagnosis of chronic intracardiac thrombi may be confounded by altered imaging characteristics due to cell necrosis and fibrous organization showing heterogeneous enhancement and possible calcification [9].
The management of intracardiac thrombi typically involves anticoagulation. Surgery is rarely necessary. However, management has to be individualized based on several factors including the size of the mass and the risk of haemodynamic instability. Histopathological evaluation is helpful due to the diagnostic difficulties and to support the decision for definitive management. This highlights the importance of a case-by-case approach to intracardiac masses.
CONCLUSION
Cardiologists need to be aware of the potential diagnostic pitfalls and therapeutic challenges in the management of right atrial masses. A multidisciplinary approach involving cardiothoracic surgery is important in the care of such patients.