ABSTRACT
Behçet's disease (BD) is multisystemic vasculitis with heterogeneous clinical manifestations. We describe the case of a 26-year-old man who presented with Budd–Chiari syndrome (BCS) related to BD. The patient received infliximab (IFX) due to the severity of vascular involvement. Subsequently, after IFX therapy, hospital-acquired pneumonia, trapped lung, and fungal infection of the lung and central nervous system developed as complications. The patient benefited from a second course of IFX and clinical remission was achieved following early identification and treatment of complications. Data on the presentation and prognosis of BCS related to BD are extremely limited. Our case report supports the growing evidence that anti-TNF antibody is a promising treatment for BD-related BCS.
LEARNING POINTS
- Behçet's disease-related Budd–Chiari syndrome is a rare form of vascular involvement that severely affects mortality.
- Behçet's disease-related Budd–Chiari syndrome is frequently confused with idiopathic thrombosis and may be underdiagnosed.
- Infliximab could be a therapeutic option for refractory Behçet's disease with major vascular involvement, but the increased risk of opportunistic infections should be kept in mind.
KEYWORDS
Behçet's disease, infliximab, Budd–Chiari syndrome, vascular involvement, bronchopleural fistula
CASE DESCRIPTION
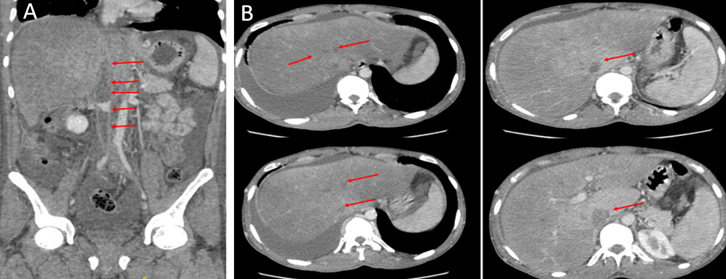
A 26-year-old Turkish man presented to the emergency department with a 7-day history of diffuse abdominal pain, nausea and vomiting. An abdominal computed tomography (CT) scan revealed extensive thrombosis involving the inferior vena cava (IVC) and bilateral main iliac veins, infrarenal, suprarenal and hepatic veins, and mild ascites. The patient underwent pharmaco-mechanical thrombectomy 12 days after presentation. An abdominal CT scan after the intervention revealed partial recanalization of the thrombosed hepatic and suprahepatic segments of the IVC, but there was no clinical improvement. After 3 months, the patient was referred to the haematology department of our centre due to a significant increase in symptoms. An abdominal CT scan showed the iliac veins and the infrarenal, suprarenal, hepatic and suprahepatic segments of the IVC were occluded right up to the right atrium junction (Fig. 1A). The portal vein was patent, but filling was absent in the hepatic veins, and drainage occurred via the azygos system and collaterals (Fig. 1B).
Figure 1. Abdominal CT scan (coronal view) showing (A) thrombosis of the infrarenal, suprarenal and hepatic segments of the inferior vena cava (red arrows) and (B) total occlusion of all hepatic veins but a patent portal vein (red arrows)
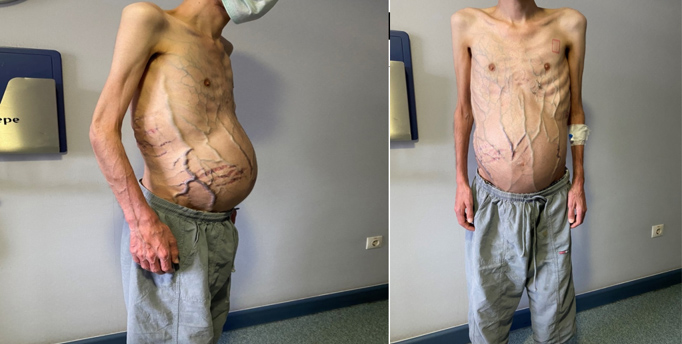
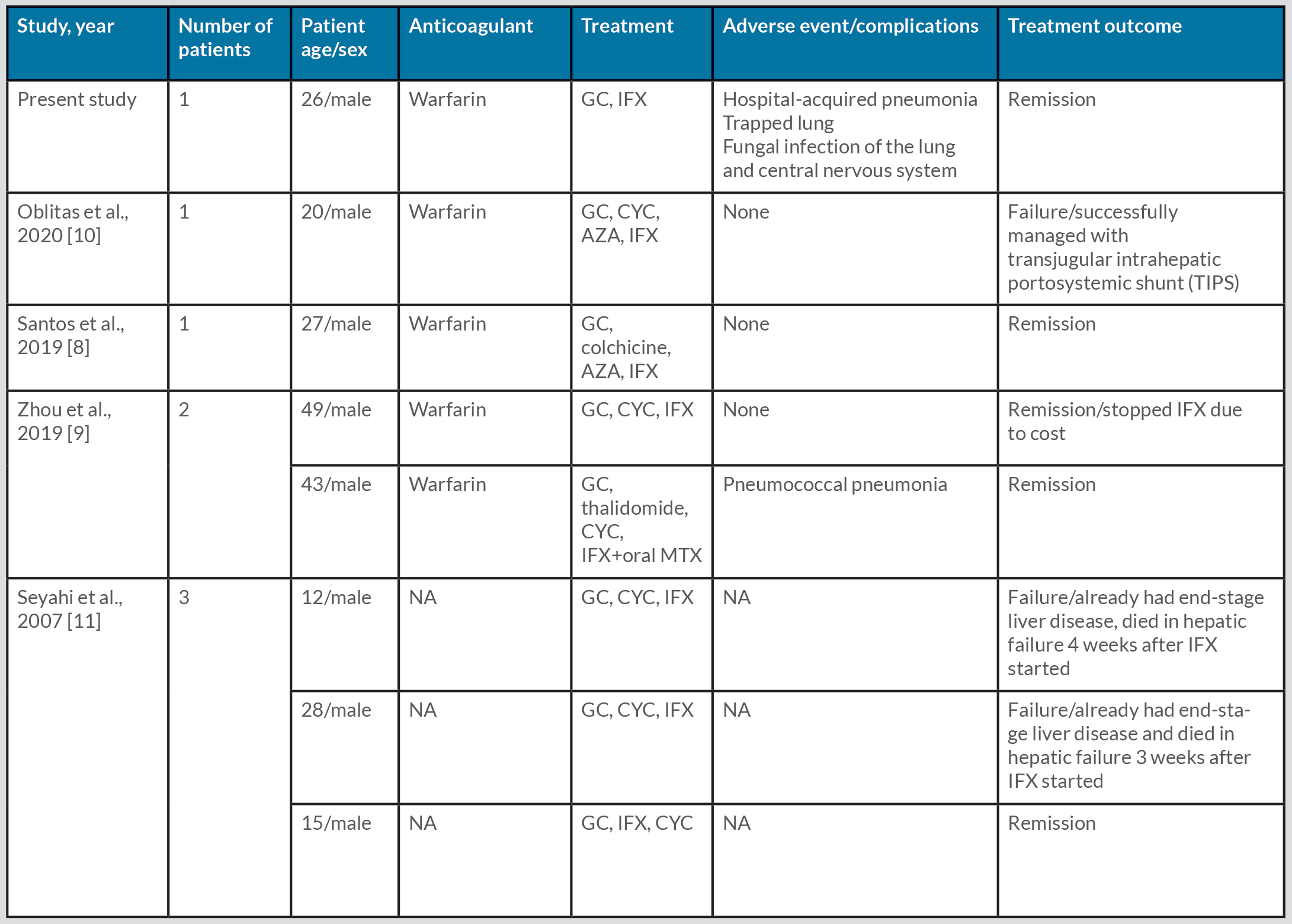
The patient was admitted to the internal medicine ward for investigation of the aetiology of Budd–Chiari syndrome (BCS). He had a history of oral aphthae (4-5 times per year), abdominal distension, pain and fatigue. Physical examination revealed ascites, diffuse superficial venous collaterals around the umbilicus (Fig. 2), and several testicular scars.
Figure 2. Findings on physical examination showed ascites, abdominal distension, and diffuse subcutaneous venous collaterals around the umbilicus, possibly due to liver insufficiency
The patient’s current medication was warfarin 5 mg daily. Laboratory tests showed haemoglobin 11 g/dl with normal mean corpuscular volume, 249,000/µl platelets, 6700/µl leukocytes, international normalized ratio (INR) 4.1, alanine aminotransferase (ALT) 267 U/l, gamma-glutamyl transferase (GGT) 56 U/l, alkaline phosphatase (ALP) 157 U/l, total bilirubin 1.5 mg/dl, urea nitrogen 30 mg/dl, ammonium 51 µmol/l (normal <50 µmol/l) and C-reactive protein (CRP) 7.7 mg/dl. Hepatitis serologies, thrombophilia panels, JAK-2 mutation, APAS antibodies, ANA, HLA-B5 and HLA-B51 were all negative. A positive pathergy test, the presence of BCS, and the history of oral and genital ulcers led to the diagnosis of Behçet's disease (BD), and 20 mg oral methylprednisolone daily was started. The patient's general condition deteriorated, and he developed hepatic encephalopathy. In a multidisciplinary discussion including gastroenterology, general surgery, rheumatology and interventional radiology, angioplasty was ruled out due to the extent of thrombosis and the technical difficulty of catheterizing the entire territory. The team decided remission through immunosuppression would have to be achieved first before a liver transplant could be offered.
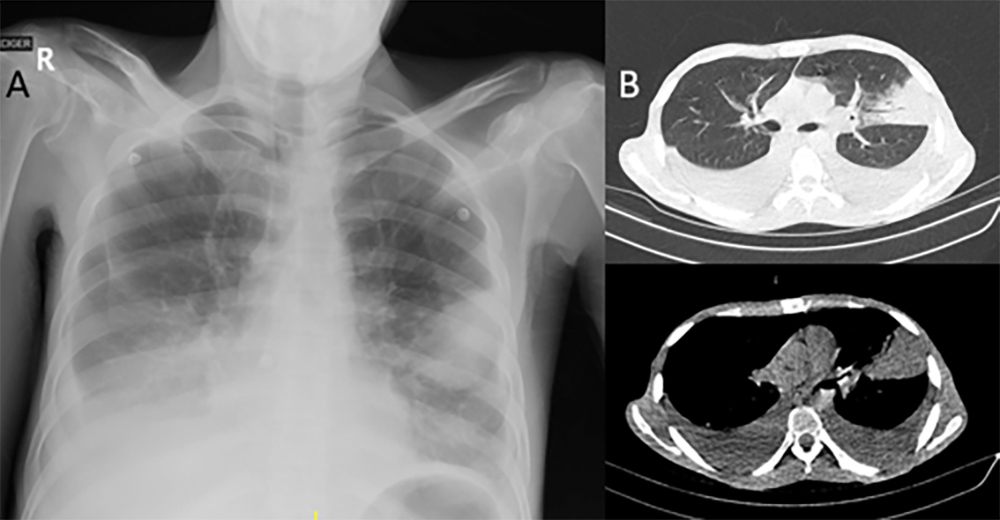
The patient received three pulses of 500 mg of intravenous methylprednisolone and continued with infliximab (IFX 5 mg/kg). After the first cycle of anti-TNF treatment, the patient developed hospital-acquired pneumonia with bilateral parapneumonic effusion, and piperacillin/tazobactam was administered (Fig. 3).
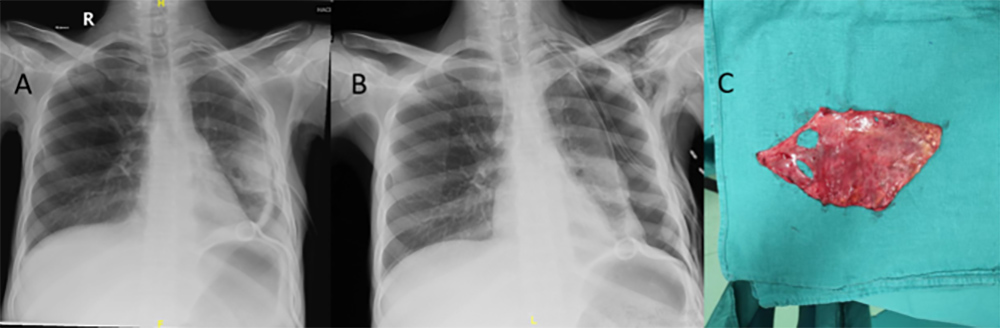
Pneumothorax developed as an iatrogenic complication after the parapneumonic effusion was sampled with a pigtail catheter (Fig. 4A). The lung did not re-expand following chest tube insertion, and persistent pneumothorax was observed (Fig. 4B). A CT scan of the thorax revealed fibrous visceral pleural thickening, trapped lung and bronchopleural fistula. Management consisted of decortication and fistula repair; Aspergillus fumigatus was isolated from the decortication material (Fig. 4C).
Figure 3. (A) Standard posteroanterior (PA) radiograph showing left pleural effusion and pulmonary infiltrate; (B) CT scan of the thorax revealing consolidation of the lingula and bilateral pleural effusion
Figure 4. (A) Pneumothorax developed as an iatrogenic complication after pigtail catheter insertion. (B) Persistent pneumothorax was observed following chest tube insertion, and trapped lung and bronchopleural fistula were diagnosed. (C) Specimen obtained following video-assisted thoracoscopic surgery with decortication
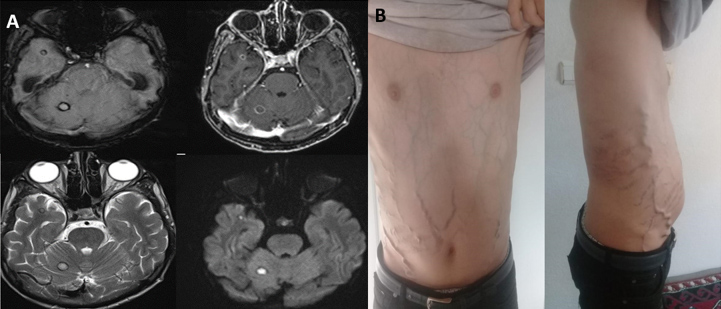
Voriconazole was added as an antifungal agent to piperacillin/tazobactam, but 1 week later, the patient suddenly developed blurry double vision and loss of consciousness. Brain MRI revealed juxtacortical lesions, one with a diameter of 8 mm, in the right cerebellar and right anterior temporal regions. Hemosiderin rings in the lesions’ periphery with limited diffusion and peripheral enhancement were observed (Fig. 5A). Granulomatous, opportunistic and fungal infections were included in the differential diagnosis. Sampling of the lesions was recommended but could not be carried out due the patient’s poor condition.
By the 44th day of voriconazole administration, the patient had no active neurological symptoms and brain MRI showed regression of the lesions. Antibiotherapy and anti-fungal therapy were discontinued after completion of the 34-day and 44-day courses of piperacillin/tazobactam and voriconazole, respectively. After three cycles of IFX (5 mg/kg) every 6 weeks, rapid clinical improvement occurred, and serum aminotransferase, total bilirubin and direct bilirubin levels decreased and returned to baseline. The patient’s symptoms resolved, and he was discharged. For the past year he has been attending regular rheumatology outpatient clinics without disease relapse (Fig. 5B).
Figure 5. (A) Brain MRI shows juxtacortical lesions, one 8 mm in diameter, with hemosiderin rings in the right cerebellar and anterior temporal regions. (B) The patient’s symptoms and ascites resolved following a dramatic response to infliximab
DISCUSSION
Behçet's disease (BD) is a relapsing-remitting vasculitis with an unknown aetiology characterized by a variety of clinical manifestations including uveitis, oral aphthae, skin lesions and genital ulcers. The neurological, gastrointestinal and vascular systems can also be affected, but less frequently [1-3]. BD-related BCS is a rare form of vascular involvement that severely increases mortality and typically involves the IVC (the suprahepatic and hepatic portions) and the hepatic veins [4]. Prognosis is poor with hepatic failure, but there is also a ‘silent’ form with a relatively better outcome [2].
Although BD is a relatively uncommon (<5%) cause of BCS in Europe, it is more frequent (9–14%) as a cause of BCS in endemic nations like Turkey [4,5]. Patients with BCS should be evaluated for BD, especially young adult male patients if they present indicative symptoms including IVC thrombosis and recurring oral or genital ulcers, as in our case [4].
The European League Against Rheumatism (EULAR) recommends a multidisciplinary approach to BD, with treatment depending on age, gender, type and severity of organ involvement [6]. Treatment for BD with vascular involvement mainly consists of immunosuppressive therapy with steroids and immunomodulators (cyclophosphamide, azathioprine and methotrexate). However, some BD patients may not respond well to these drugs. Monoclonal anti-TNF antibodies could be considered for treatment-refractory patients with major vessel involvement [6].
Recently, a multicentre study including 61 patients with BD-related BCS showed a significantly higher mortality rate (p = 0.04) in the 25 (54.3%) patients with IVC thrombosis [4]. In another study with a large cohort of 9000 patients with BD, 43 had BCS. The mortality rate for symptomatic patients with ascites was 60%, with a median survival of 10 months following diagnosis [7]. In comparison, asymptomatic patients without ascites who gradually developed BCS had a better prognosis with a mortality rate of <10% at 7 years. Three treatment-refractory patients were treated with IFX, which was successful in reducing disease activity in only one of these patients as the other two had end-stage liver disease and eventually died from liver failure [7]. Another case report has described a patient with BD-related BCS similar to our case. The patient had suprahepatic vein thrombosis refractory to azathioprine and corticosteroids, but complete remission was observed 3 months after the start of IFX therapy [8]. A study describing two patients with BD-related BCS from China who responded well to IFX, reported one patient developed pneumococcal pneumonia during follow-up [9]. Another case report described severe BD-related BCS, where thrombosis progressed despite anticoagulant and immunosuppressive therapy, including with IFX. However, the patient was successfully treated with a transjugular intrahepatic portosystemic shunt (TIPS) [10] (Table 1).
Table 1. Clinical characteristics of Behçet’s disease-related Budd–Chiari syndrome treated with anti-TNF antibodies
AZA, azathioprine; CYC, cyclophosphamide; GC, glucocorticosteroids; IFX, infliximab; MTX, methotrexate; NA, not available.
The role of interventional techniques in the management of BD-related BCS remains controversial. The possibility of vascular pathergy phenomena caused by venous manipulation should be taken into account. The EULAR guidelines do not provide any clear suggestions in this area [10].
The second pillar of therapy for BD with vascular involvement is anticoagulation. However, as in our case, the use of anticoagulant therapy is still uncertain in BD-related BCS patients with residual liver insufficiency [4]. Thrombi in BD are firm and adhesive and carry a low risk of embolization. Additionally, the potential for pulmonary arterial aneurysms is another reason to avoid anticoagulation [6]. EULAR recommend steroid and immunosuppressive therapy while discouraging the use of anticoagulants [6].
In conclusion, we present a rare case of BD-related BCS with a dramatic response to IFX. Although BD is a well-known disease, data on the outcome of BD-related BCS are very limited in the literature. Our case report shows IFX was successfully used to treat BD-related BCS, but the increased risk of opportunistic infections should be kept in mind. Early and aggressive treatment with vigilant monitoring of complications is needed to decrease mortality caused by this life-threatening disorder.