ABSTRACT
Introduction: Percutaneous embolectomy using the percutaneous large-bore aspiration embolectomy FlowTriever system (INARI Medical, Irvine, CA, USA) is a promising method for reducing high clot burden in patients with intermediate- to high-risk pulmonary embolism (PE).
Case Description: A 45-year-old woman with intermediate- to high-risk PE underwent percutaneous intervention using the FlowTriever retrieval/aspiration system. After the procedure, she experienced several cardiac arrests from pericardial tamponade and required pericardiocentesis, leading to haemodynamic stabilization.
Discussion: To our knowledge, this is the first documented case of the use of the FlowTriever system causing micro-perforation of the right ventricle, resulting in tamponade and cardiac arrest.
Conclusion: Percutaneous embolectomy has shown promise results in reducing clot burden and improving haemodynamic stability but has risks and limitations and requires specialized knowledge and training. In addition, more data are required from centres using the FlowTriever system to ensure adequate training and safety.
LEARNING POINTS
- Percutaneous mechanical thrombectomy using the FlowTriever or similar devices can be paramount in reducing morbidity and mortality from an intermediate- to high-risk pulmonary embolism given the immediate improvement in haemodynamics that cannot be achieved by anticoagulation alone.
- Cardiac micro-perforation is a potential complication of catheter-based embolectomy devices such as FlowTriever and should be suspected in the setting of pericardial effusion following the procedure.
KEYWORDS
Cardiac tamponade, percutaneous pulmonary embolectomy, FlowTriever retrieval/aspiration system, micro-perforation
INTRODUCTION
Pulmonary embolism (PE) is the third most common cause of cardiovascular mortality in the USA, with 60,000–100,000 deaths annually from PE after myocardial infarction and stroke [1]. For several decades, the mainstay of treatment has been anticoagulation. However, more aggressive alternatives such as surgical embolectomy and systemic thrombolysis have been used for high-risk PE. More recently, catheter-based interventions have revolutionized the management of PE. After the FLARE (FlowTriever Pulmonary Embolectomy Clinical Study) trial, percutaneous embolectomy using the FlowTriever system (INARI Medical, Irvine, CA, USA) has received attention as regards intermediate- to high-risk PE [2] and has emerged as a promising method of reducing clot burden in such patients [3]. However, while the intervention has been widely accepted worldwide, it is associated with various peri-procedural complications and adverse effects. We present a case of cardiac tamponade as a procedural complication of this novel approach which has not been reported previously in the literature.
CASE DESCRIPTION
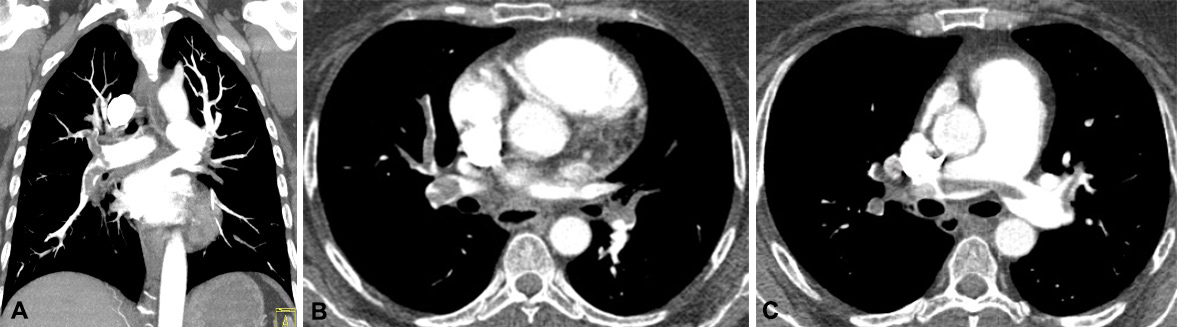
A 45-year-old woman with a medical history of rheumatoid arthritis presented with new onset dizziness, palpitations, mild dyspnoea and dry cough. Upon arrival, she was tachycardic with a heart rate of 148 bpm, tachypnoeic with a respiratory rate of 28/min, and normotensive with a blood pressure of 118/95. An electrocardiogram (ECG) revealed sinus tachycardia with ST segment depression in precordial leads V3–V5. Her troponin T level was 42 ng/ml. Pro-brain natriuretic peptide (pro-BNP) on presentation was 30 pg/ml (reference range 0–299 pg/ml). D-dimer was elevated at 10.32 feU/ml (reference range 0–0.5 feU/ml). Computed tomography angiography of the chest showed extensive PE involving all lobes of both lungs, including saddle embolus, and imaging findings consistent with right heart strain (RHS) (Fig. 1). The patient was started on a continuous infusion of intravenous (IV) unfractionated heparin and taken to the catheterization laboratory for mechanical thrombectomy.
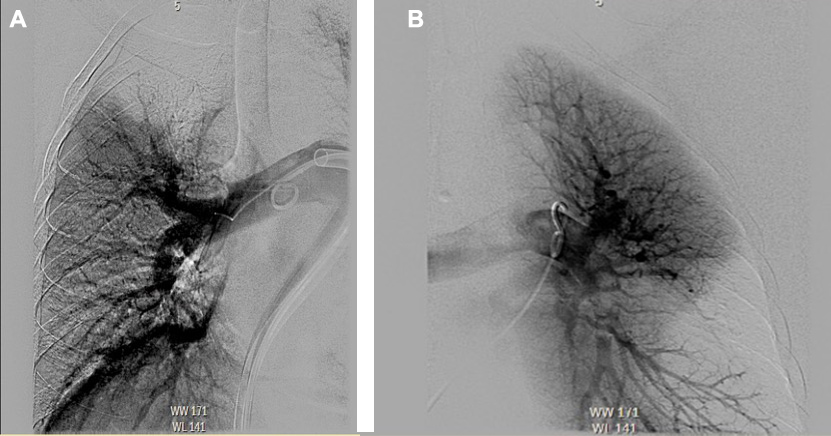
Mechanical thrombectomy of the right upper and lower lobes, the right main pulmonary artery (PA), and the left lower lobe was performed using the FlowTriever device (Fig. 2), following which there was a significant improvement in mean PA pressure, which decreased from 42 mmHg to 19 mmHg. The patient was admitted to the medical intensive care unit (MICU).
Figure 1. Computed tomography (CT) images showing an extensive pulmonary embolus burden, including saddle embolus, and thrombus in the main pulmonary arteries. (A) Coronal view with saddle embolus and large thrombus burden in the right main pulmonary artery extending into lobar and segmental branches. (B) Axial view showing nearly occlusive emboli in bilateral pulmonary trees. (C) Axial view showing saddle embolus
Figure 2. Pulmonary angiogram during catheter-based embolectomy using the FlowTriever device. (A) Right-sided pulmonary angiogram showing decreased perfusion in the right upper lobe. (B) Left-sided pulmonary angiogram showing decreased perfusion in left lower lobe
However, 30 minutes after admission to the MICU, the patient became acutely hypotensive. A transthoracic echocardiogram (TTE) revealed a new moderate-sized pericardial effusion with clinical evidence of pericardial tamponade physiology. A total of 400 ml of bloody pericardial fluid was removed via emergent pericardiocentesis and a pericardial drain was left in place. Post-procedure TTE showed resolution of the pericardial effusion. Approximately 8 hours later, the patient experienced a pulseless electrical activity cardiac arrest. The advanced cardiac life support (ACLS) protocol was initiated, and the pericardial drain was opened, resulting in the return of spontaneous circulation (ROSC). Emergent bedside TTE showed a moderate-sized pericardial effusion had reaccumulated with right ventricle (RV) collapse. A total of 500 ml of bloody fluid was removed from the pericardial drain. Protamine sulfate was administered for the reversal of heparin, and the patient received several units of blood products for haemorrhagic shock. She was subsequently weaned off pressor support over next 48 hours. Four days later, the pericardial drain was removed, and 1 day after that, the patient was extubated. Pericardial effusion was ultimately thought to be due to micro-perforation of the RV that likely was spontaneously sealed off.
DISCUSSION
In the USA, nearly a third of hospitalized patients are at risk of developing venous thromboembolism (VTE), and up to 600,000 cases of VTE are diagnosed annually, with 100,000 associated deaths [4]. Interestingly, over the past two decades, there has been little change in the mortality rate of PE, making this an appealing area for innovation and technology [5]. Aside from anticoagulation, which is the current treatment mainstay, other management avenues include systemic thrombolysis, catheter-directed intervention, and surgical embolectomy. Although systemic thrombolysis has shown benefits in randomized trials for intermediate- and high-risk PE, it has been associated with an increased risk of significant bleeding and intracerebral haemorrhage, limiting its use in clinical practice [1]. As a result, percutaneous catheter-based interventions are frequently used and appropriate if systemic thrombolysis is contraindicated or urgent recanalization of PE is warranted [1, 3]. Furthermore, in PE, clot extraction reduces clot burden, independent of any potential contraindications to thrombolysis[6]. The primary aim of catheter-based clot retrieval is to expeditiously alleviate the physiological burden of PE by reducing RV afterload and improving RV function to allow recovery of systemic blood pressure and oxygenation [7].
Interventional options include (i) thrombus fragmentation with a pigtail or balloon catheter, (ii) rheolytic thrombectomy with hydrodynamic catheter devices, (iii) suction thrombectomy with aspiration catheters, and (iv) rotational thrombectomy [1, 3]. The three primary interventional devices approved by the US Food and Drug Administration for marketing include the EkoSonic endovascular system, FlowTriever embolectomy device, and Indigo thrombectomy system [1]. Our patient underwent urgent percutaneous mechanical thrombectomy using the FlowTriever system for a saddle PE with RHS. The FlowTriever system is a large-bore 20F catheter that is advanced through the occluded pulmonary artery and then retracted while mechanically engaging and retrieving the thrombus by deploying three self-expanding nitinol disks while the catheter aspirates the clot [1]. The device was approved after the FLARE trial, a prospective, multicentre randomized controlled trial that enrolled 106 patients at 18 US sites between April 2016 and October 2017 [2]. The primary endpoint of change in the RV/LV ratio from baseline to 48 hours decreased by an average of 0.38 (p<0.0001) after the procedure [2]. Another systematic review of 348 patients using various percutaneous clot retrieval techniques reported a success rate (defined as improvement in haemodynamic parameters immediately after the procedure) of over 80% in patients with acute massive PE [8].
However, notwithstanding its success and low risk of adverse events, randomized controlled trials are limited to a few studies [1, 7]. Despite the large-bore design to capture large and chronic thrombi, the system also introduces the risk of bleeding and vascular access complications. Other potential complications include arrhythmias, bleeding, pulmonary arterial dissection/perforation, atrial or ventricular perforation causing tamponade, and distal embolization of fragmented thrombi [9]. In the systemic review by Skaf et al., minor bleeding at the insertion site occurred in 8% of all patients including those with and without thrombolytic therapy [8]. Major bleeding at the insertion site occurred in only 2% of the total cases. One patient experienced RV perforation with the Greenfield catheter, and no patients had PA perforation [6].
In the FLARE trial, there were no instances of cardiac injury and only one instance of pulmonary vascular injury, which was adjudicated as procedure-related and not device-related [2]. Notably, despite the large-calibre venous access required for the FlowTriever, there were no instances of device-related vascular injury. Although results so far are promising, additional research is needed to optimize this device's use and define the optimal patients and timing for intervention.
Our case demonstrates the real-world risks of using these devices and highlights the need for increased studies and for operator training and specialization to minimize adverse outcomes. Unfortunately, prospective data on these devices are limited, and as it is difficult to conduct studies in these patient populations, it is important that real-world data be collected from centres that regularly use these devices so that there can be better assessment of the risks involved and outcomes for patients can be improved.