ABSTRACT
Tacrolimus is commonly used as a prophylactic against acute rejection in transplant patients. Tacrolimus toxicity has numerous presentations that have been well documented in the literature and can be induced by a wide variety of agents. We present a novel case of tacrolimus toxicity in a cardiac transplant patient induced by outpatient treatment for COVID-19 pneumonia with ritonavir-nirmatrelvir, which was successfully treated with phenytoin therapy.
LEARNING POINTS
- Ritonavir-nirmatrelvir is a newly approved antiviral therapy for COVID-19 to prevent hospitalization and is increasingly prescribed in the outpatient setting.
- Thorough assessment of drug interactions prior to starting ritonavir-nirmatrelvir can prevent tacrolimus toxicity in patients with solid organ transplants.
- Phenytoin increases the metabolism of tacrolimus and can be safely utilized to treat tacrolimus toxicity.
KEYWORDS
COVID-19, tacrolimus, heart transplantation, Paxlovid, phenytoin
INTRODUCTION
Patients with cardiac and other solid-organ transplants on chronic immunosuppressive therapy should undergo rigorous drug–drug interaction screening prior to initiation of therapy. We describe a patient on chronic immunosuppression with tacrolimus who was initiated on a novel regimen for outpatient treatment of COVID-19 pneumonia with a negative outcome. Care must be taken in prescribing novel drugs, particularly in the setting of a high risk of a drug–drug interaction with ongoing tacrolimus use. In such a setting, consider phenytoin for acute reversal of tacrolimus toxicity.
CASE DESCRIPTION
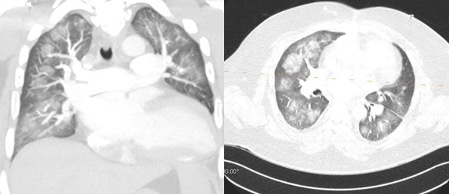
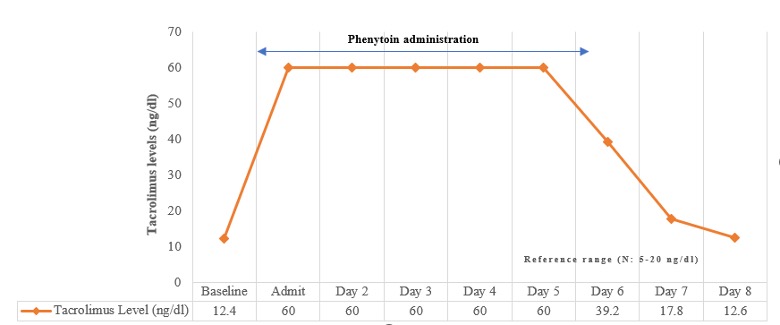
We present the case of a 43-year-old man with a medical history significant for insulin-dependent diabetes mellitus, obstructive sleep apnoea, chronic kidney disease stage 3A, and non-ischaemic cardiomyopathy following an orthotopic heart transplant due to viral myocarditis. The patient presented with worsening cough, dyspnoea and haemoptysis after being initiated on Paxlovid (ritonavir and nirmatrelvir) therapy as an outpatient. He had not been previously vaccinated against SARS-CoV-2 and tested positive for COVID-19 pneumonia within 1 week of arrival. Laboratory results were concerning for hyperkalaemia, normal anion-gap metabolic acidosis, acute kidney injury on chronic kidney disease, stable chronic pancytopenia, and tacrolimus levels >60 ng/ml (normal level: 5–20) increased from 11 ng/ml before treatment. He required oxygen supplementation after admission for ongoing hypoxia. CT imaging showed evidence of extensive bilateral ground-glass opacities (Fig. 1), concerning for alveolar haemorrhage in the setting of coagulopathy and COVID-19 pneumonia, which was treated with inhaled tranexamic acid. Extensive microbiological work-up for other bacterial, fungal and viral causes was negative. Tacrolimus toxicity as a result of ritonavir-nirmatrelvir therapy was presumed to be the primary cause of illness. The patient was admitted to the medical intensive care unit and was treated with phenytoin to increase the metabolism of tacrolimus with significant improvement. The patient’s tacrolimus levels improved to 12.6 ng/ml (Fig. 2) and he was discharged home on room air within 2 weeks of initial admission.
Figure 1. CT imaging findings of ground-glass opacities attributed to diffuse alveolar haemorrhage in the setting of coagulopathy in a patient with COVID-19 and tacrolimus toxicity
Figure 2.Trend in tacrolimus level with phenytoin treatment (in ng/dl) over an 8-day period
DISCUSSION
Drug–drug interactions in heart transplant patients are an increasing cause for concern in the setting of concomitant disease, environmental exposures, high risk of infection (as in our patient), and even the narrow therapeutic index for most immunosuppressive therapies. One such drug, tacrolimus, is widely used as a prophylactic agent to prevent solid organ rejection after transplantation. It specifically binds to FK605 binding protein and blocks T-cell proliferation, and is primarily excreted through bile. Given its narrow therapeutic index, it has a wide range of adverse effects [1, 2]. Tacrolimus is metabolized by the liver via cytochrome (CYP) P3A4, hence any CYP3A4 inhibitor such as protease inhibitors (ritonavir), can lead to an increased toxic concentration of tacrolimus. Hence prescription of CYP3A4 inhibitors should be avoided in patients who are taking tacrolimus. In case of co-administration of CYP3A4 inhibitors and tacrolimus, serial monitoring of tacrolimus levels is integral to prevent tacrolimus toxicity [3].
Additionally, we recommend that medication reconciliation and close evaluation of current immunosuppressive therapies should be considered prior to initiating any new medication in solid organ transplant patients even though it might be useful in the greater population. In this particular instance, it was thought the combination of oral ritonavir and nirmatrelvir, an investigational protease inhibitor by Pfizer, would be beneficial as it has been shown in pharmaceutical trials to reduce the risk of hospitalization or death by 89% [4]. Drug–drug interactions can be easily avoided by using freely available COVID-19 drug interaction checkers [5].
Acute tacrolimus toxicity can present in a wide variety of ways and usually with mild symptoms such as nausea, headache, electrolyte changes and elevated serum creatinine [2]. Most methods to treat tacrolimus toxicity are ineffective. Occasionally, phenytoin is used to clear acute overdose [6, 7]. We were able to successfully treat tacrolimus toxicity by stopping Paxlovid therapy and using phenytoin as a treatment option. Of note, pulmonary manifestations of tacrolimus toxicity are very rare and may include diffuse interstitial lung disease. In our patient, the pulmonary effects were likely in the setting of alveolar haemorrhage with COVID-19-related coagulopathy. The notable limitation in our case was that our laboratory does not report tacrolimus values above 60 ng/dl, so such all values >60 ng/dl were reported as 60 ng/dl for analysis purposes.
To our knowledge, this is the first description of ritonavir and nirmatrelvir-induced tacrolimus toxicity in an immunocompromised heart transplant patient. The differential diagnosis includes pulmonary manifestations of tacrolimus toxicity, although this is less likely in view of its rarity. Additional considerations include slightly increased drug levels given the decreased levels of CYP enzymes in acute infection, including COVID-19, since tacrolimus is metabolized almost entirely by liver and intestinal CYP3A enzymes [8]. Our case is rare both because of the novel finding of ritonavir and nirmatrelvir-induced tacrolimus toxicity and also because it is one of the few documented cases of successful treatment with phenytoin of acute tacrolimus toxicity.
Medication reconciliation is very important in all patients to prevent adverse drug reactions. Although major adverse drug events (ADEs) account for up to 16% of emergency department visits, data on reducing ADEs by medication reconciliation are rare [9]. Nevertheless, both hospital and community changes in medication should be monitored closely in solid organ transplant patients to prevent drug–drug interactions. Given the cumulative complications of drugs with a narrow therapeutic index such as tacrolimus, one should closely monitor its activity and any potential for drug–drug interactions such as acute tacrolimus toxicity as described in our patient.
CONCLUSION
Caution should be exercised and careful monitoring for drug–drug interactions should be conducted when prescribing ritonavir and nirmatrelvir as outpatient therapy for COVID-19 pneumonia in all patients, but especially in transplant patients on chronic immunosuppression with tacrolimus. When acute tacrolimus toxicity is seen in such cases, treatment with phenytoin can be considered.