ABSTRACT
Lithium is a medication commonly used as a mood stabilizer and can have numerous long-lasting side effects. In this case report, we aim to remind clinicians of such consequences. A 68-year-old woman with a psychiatric history presented for mild COVID-19 and developed sinus bradycardia. A permanent pacemaker was planned for her but was cancelled following good history-taking which revealed prior lithium use. The patient was found to have hyperparathyroidism and hypothyroidism, treatment of which resolved the bradycardia. This case serves to remind clinicians that history-taking remains of paramount importance as in this scenario of bradycardia in a psychiatric patient. An invasive therapeutic measure was precluded by good history-taking. There are several mechanisms by which hypothyroidism and hyperparathyroidism can induce bradycardia. COVID-19 infection can also induce bradycardia.
LEARNING POINTS
- Clinicians should suspect lithium as a cause in any psychiatric patient with new-onset bradycardia.
- Lithium can induce bradycardia either directly or even after discontinuation indirectly through hypothyroidism and/or hyperparathyroidism.
- Invasive measures can be avoided with adequate management of these endocrine issues.
KEYWORDS
Lithium, bradycardia, pacemaker, hyperparathyroidism, hypothyroidism
CASE DESCRIPTION
A 68-year-old female patient presented to the emergency department (ED) for recent behavioural changes while living in a group home. It was reported that she was combative and agitated. In the ED, she was calm but refused to participate in examinations or to answer questions. Vital signs were stable with a heart rate of 50–70 beats per minute (bpm). Review of her medical records revealed a history of schizoaffective disorder and bipolar disorder on risperidone.
Basic laboratory work showed hypercalcaemia at 12.5 mg/dl with elevated creatinine at 1.12 mg/dl and eGFR of 50 ml/min from a baseline of normal renal function. Electrocardiography (EKG) showed sinus rhythm. The patient tested positive for COVID-19 but remained asymptomatic and on room air throughout the entire hospitalization. She was admitted to the COVID isolation unit and risperidone was restarted. She was refusing to eat or occasionally to take medications. She was maintained on IV fluid infusion for dehydration.
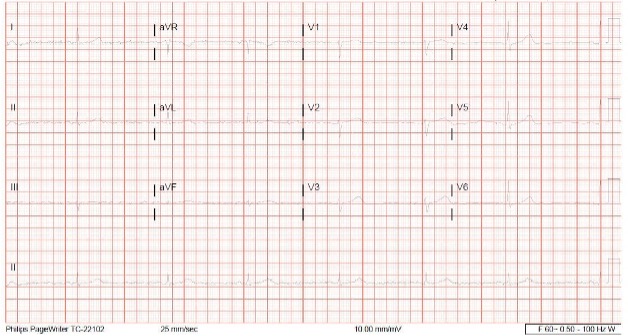
Five days into hospitalization, telemetry showed persistent sinus bradycardia as low as 26 bpm while the patient was apparently asymptomatic and blood pressure was at an acceptable level (Fig. 1). This bradycardia was initially believed to be secondary to risperidone. As the patient was refusing to engage in conversation, it was difficult to assess if the bradycardia was truly asymptomatic and to assess for chronotropic incompetence. Although oral risperidone was mostly refused, she did receive two doses but awareness of a temporal relationship with the onset of bradycardia prompted discontinuation of the medication. However, no improvement in her bradycardia was noticed.
Although pacemaker placement was initially planned for the patient, further history from her psychiatrist revealed that she had been taking lithium for several years and it had been replaced with risperidone recently in the last few months. This prompted checking for hyperparathyroidism and hypothyroidism. Parathyroid hormone (PTH) was high while thyroid hormones were low and thyroid hormone replacement was started. Despite fluid maintenance, her calcium levels kept waxing and waning between 12 and 11 g/dl. Hypercalcaemia treatment was escalated and she was given IV zoledronate and calcitonin that gradually prompted improvement in her heart rate. Pacer pads were in place but were not required. Improvement in heart rate above the bradycardic range with a continuous decline in calcium levels and thyroid hormone treatment ruled out the need for a pacemaker.
Psychiatric symptoms improved with treatment of hypercalcaemia and hypothyroidism, and the patient was accepting oral medications. Re-challenge with risperidone was not associated with bradycardia. The patient was discharged to a psychiatric unit after she completed an isolation period. No further episodes of bradycardia were recorded during a second admission to the psychiatric unit a few months later.
DISCUSSION
History-taking remains essential in medical practice even with the most recent advances. Bradycardia in this patient was likely mainly induced by the synergistic effects of hyperparathyroidism and hypothyroidism. This is based on the observation that bradycardia resolved rapidly within days after the correction of hypercalcemia and hypothyroidism.
The mechanism behind bradycardia induced by hyperparathyroidism is unclear, but there are several proposed theories. The observation that exogenous PTH induces necrosis of rat myocytes raises the possibility that hyperparathyroidism may directly induce SA node dysfunction [1, 2]. In addition, hyperparathyroidism can also cause hypercalcaemia, which is associated with prolongation of the PR segment and the QRS interval and hence shortening of the QT interval, which is usually associated with bradycardia [3]. Some case reports suggested AV node dysfunction secondary to chronic severe hypercalcaemia in malignancy patients [4, 5]. Reversible AV node dysfunction in acute hypercalcaemia was suggested by a previous case report, although in this scenario the mechanism is less clear [5].
On the other hand, hypothyroidism can per se induce bradycardia. Indirectly, hypothyroidism can lead to diastolic hypertension and hence reflex bradycardia. Also, hypothyroidism can induce bradycardia at a genetic level by regulating pacemaker-related genes through transcription as well as the beta-adrenergic system in cardiomyocytes [6].
A role for COVID infection in this patient’s bradycardia cannot be ruled out. In fact, one major retrospective review of the cardiac complications in hospitalized COVID-19 patients reported that 8% of patients developed significant sinus bradycardia, while another 8% developed complete heart block involving either first- or second-degree AV block [7]. The mechanism is also not clear. In a small retrospective study, a relationship was found between cytokine storm, especially interleukin-6, and bradycardia, and it was suggested that bradycardia or even relative bradycardia may herald the storm [8].
Lithium was an unlikely cause of bradycardia in our patient as it had been discontinued several months prior to presentation, but it is worth mentioning that lithium can induce sinus node dysfunction [9]. Possible mechanisms include dose-dependent inhibition of myocyte voltage-gated sodium channels that decreases intracellular potassium [9, 10].