ABSTRACT
Introduction: Haemolysis, elevated liver enzymes and low platelet count (HELLP) syndrome is a leading cause of maternal mortality. The emergence of coronavirus disease 2019 (COVID-19) has led to challenges in diagnosing HELLP syndrome due to overlapping clinical and laboratory presentations. We report a case of HELLP syndrome complicated by COVID-19 infection.
Case Description: An otherwise healthy pregnant 31-year-old woman presented with fever, myalgia and headache. She was found to be COVID-positive with laboratory signs of HELLP syndrome. Symptoms and laboratory findings trended toward normal after delivery confirming the diagnosis of HELLP syndrome.
Discussion: A prompt diagnosis of HELLP syndrome is essential to avoid maternal and fetal complications. Clinicians should be aware of the similarities in presentation between HELLP syndrome and COVID-19 for timely diagnosis and treatment.
LEARNING POINTS
- SARS-CoV-2 preferentially binds to ACE2 which is expressed in extrapulmonary tissue including placental tissue.
- COVID-19, HELLP syndrome and preeclampsia may have similar characteristics including elevated blood pressures, liver dysfunction, cardiopulmonary complaints and hypercoagulability.
- The temporal relationship of symptomatic improvement with delivery and after delivery may better differentiate HELLP syndrome from COVID-19.
KEYWORDS
COVID-19, SARS-CoV-2, preeclampsia, HELLP, gestational hypertension
INTRODUCTION
HELLP syndrome is a severe complication of gestational hypertension that manifests with the triad of haemolysis due to microangiopathic haemolytic anaemia, elevated liver enzymes and low platelets [1]. The COVID-19 pandemic has introduced additional challenges in diagnosing HELLP as COVID-19 infection can cause other systemic complications including elevated blood pressure, haemolysis, thrombocytopenia, kidney injury and transaminitis [2–4] mimicking preeclampsia and HELLP features.
While the pathology of COVID-19 appears to be multifactorial, it is postulated that angiotensin-converting enzyme 2 (ACE2) mediates the action of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) in the regulation of the renin-angiotensin system, platelet activation and thrombosis, endothelial dysfunction and arterial pressure [3, 5]. ACE2 is highly expressed in placental tissue including syncytiotrophoblasts, endothelium, cytotrophoblast and vascular smooth muscle of the villi [3]. To that end, recent reports in pregnant patients with COVID-19 show evidence of maternal vascular malperfusion (MVM), vascular injury and thrombi [6-8]. Similar pathophysiological mechanisms including platelet activation and endothelial dysfunction have also been reported in HELLP syndrome and preeclampsia [5]. It is unclear how these pathophysiological mechanisms interact or if there is synergism between the two aetiologies.
We present a case of HELLP syndrome in a patient who had their clinical course complicated by SARS-CoV-2 infection. We then discuss the challenges faced by clinicians in differentiating SARS-CoV-2 infection and HELLP syndrome.
CASE DESCRIPTION
A previously healthy 31-year-old G3P2 female presented to the emergency department at 32, 4/7 weeks gestation, complaining of fever, headache and myalgia that had started overnight. She reported no history of hypertension, diabetes mellitus or other chronic medical conditions. She was not taking medications and denied a history of hepatitis or drug use. Obstetric history revealed two spontaneous vaginal deliveries at term without complications. She reported normal fetal movements and no leakage of fluid, contractions or vaginal bleeding. The patient denied abdominal pain, dyspnoea, chest pain, headache or vision changes. She was found to be positive for SARS-CoV-2 but was saturating well on room air. She denied receiving any vaccine for SARS-CoV-2 or having past COVID-19 infections.
On examination, the patient was afebrile (36.8°C), respiratory rate was 18 bpm, pulse was 75–90 bpm, and she had elevated blood pressures of 141–169/83–89. Cardiopulmonary, genitourinary and abdominal physical examinations were unremarkable. There were no signs of preterm labour: ultrasound showed a closed cervix and a normal cervical length (3 cm). The cervix was firm and posterior with 50% effacement and a –3 station. In fetal assessment, the patient had a reactive non-stress test and a biophysical profile of 8/8. Laboratory results were notable for mild thrombocytopenia and transaminitis (Table 1). The hepatitis panel was negative. Due to elevated blood pressures, differential diagnoses included a stress reaction secondary to SARS-CoV-2 infection versus preeclampsia with severe features.
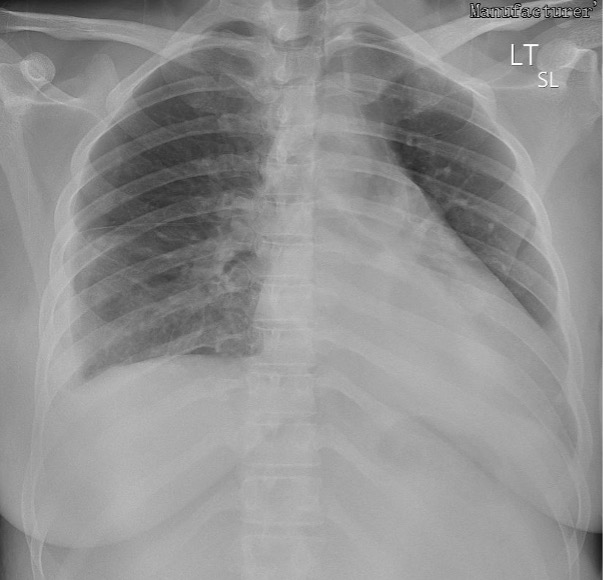
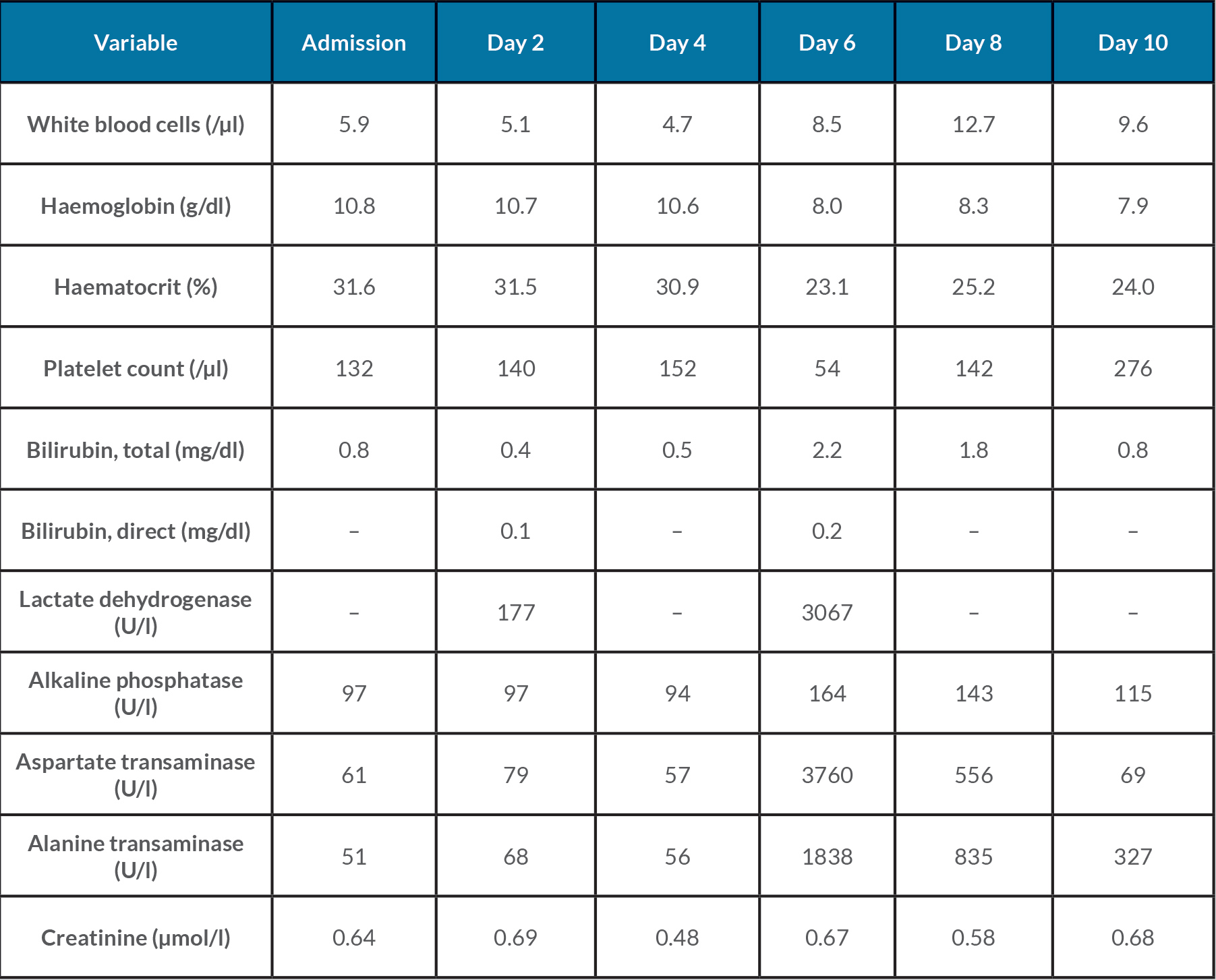
The patient was admitted for observation. A 24-hour urine protein collection was ordered to rule out preeclampsia, eventually yielding 368 mg of protein. On day 2 of admission, the patient had occasional blood pressures in the severe range (162–174/80–82) that responded well to IV labetalol. Magnesium sulfate and labetalol 200 mg by mouth twice a day were started. The patient remained stable, and the plan was to deliver at 34 weeks if blood pressures remained well controlled. On day 4, she was noted to have severe blood pressures (142–167/86–98) refractory to antihypertensives. A decision was made to proceed with a caesarean section to avoid maternal and fetal complications. The patient tolerated the procedure well. A chest x-ray revealed bilateral bibasilar patchy opacities, which were reported to be consistent with viral pneumonia (Fig. 1). Repeat laboratory results showed significant thrombocytopenia, worsening transaminitis and elevated lactate dehydrogenase on day 6 (Table 1), and decreased haptoglobin (<15 mg/dl) on day 7 consistent with an exacerbation of HELLP syndrome, as opposed to sequelae of SARS-CoV-2 infection. A microscopic examination of the placenta revealed a small 0.6 cm infarct with no other obvious abnormality. Within 72 hours after delivery, laboratory values began normalizing. The patient was discharged on day 10 with plans to follow up at an outpatient clinic in 1 week.
Figure 1. Chest x-ray (anterior-posterior view) revealing bilateral bibasilar patchy opacities
Table 1.Laboratory data throughout the hospital course
DISCUSSION
We report a case of HELLP syndrome complicated by COVID-19 infection in the third trimester of pregnancy leading to caesarean section and preterm delivery at 33, 1/7 weeks of gestational age. Our patient presented with non-specific symptoms and a positive SARS-CoV-2 real-time polymerase chain reaction (RT-PCR) test without any signs of respiratory distress. The diagnosis of preeclampsia with severe features and associated HELLP syndrome was confirmed only after the caesarean section when the patient’s blood pressure and laboratory values began normalizing. This case adds to the growing body of evidence that there is considerable overlap in the clinical and laboratory presentations of the two aetiologies, which can complicate the timely diagnosis of HELLP syndrome in the setting of SARS-CoV-2 infection.
Due to the similarities between the two aetiologies, it is essential for clinicians to focus on several clinical features that may aid in differentiating between HELLP and COVID-19. Elevated blood pressures and proteinuria are more suggestive of HELLP syndrome [5] as 4–12% of women diagnosed with preeclampsia will develop this triad [9]. However, due to atypical normotensive presentations of HELLP, the absence of elevated blood pressures and proteinuria does not preclude the diagnosis [5]. Features that favour a COVID-19 diagnosis may include those that highlight an infectious aetiology such as fever and leucocytosis in addition to symptoms of hypoxia and respiratory distress. These clues can be complemented by imaging including a chest x-ray or CT scan and other screening parameters such as D-dimer which, however, may have limited sensitivity in pregnancy. Angiogenic markers including maternal levels of sFlt-1/PlGF have also demonstrated some clinical utility as they can help rule out suspicions of preeclampsia [10]. Ultimately, the diagnosis appears best confirmed after delivery as HELLP begins to resolve 24–72 hours after delivery compared with COVID-19 where the timing of birth does not matter [5] as demonstrated in our case.
While our understanding of COVID-19 concerning maternal–fetal health is incomplete, pregnant patients with concurrent COVID-19 may be at an increased risk for complications. Thus, vigilant screening of SARS-COV-2 infection in pregnant patients is pertinent for appropriate care.
CONCLUSION
Physicians should be aware of the risk of secondary nocardiosis especially in cases of severe COVID-19, and they should prescribe relevant bacteriological examinations in case of secondary deterioration of respiratory status among COVID-19 patients, especially when secondary infection is suspected.