ABSTRACT
Introduction: mRNA COVID-19 vaccines are very safe, but rare adverse events such as transverse myelitis have been reported after COVID-19 vaccination.
Case Description: We report the case of 50-year-old man who presented with progressive lower extremity weakness, back pain and urinary retention after his second dose of the Pfizer COVID-19 vaccine. MRI of the spine revealed longitudinally extensive transverse myelitis (LETM). He recovered completely after treatment with intravenous methylprednisone and physical therapy.
Discussion: This case highlights the rare association between LETM and COVID-19 vaccines and encourages clinicians to maintain a high index of suspicion for prompt diagnosis and treatment.
LEARNING POINTS
- Longitudinally extensive transverse myelitis (LETM) is rare adverse events after mRNA COVID-19 vaccination.
- Clinicians should maintain a high index of suspicion for prompt diagnosis of vaccine-induced transverse myelitis.
- Vaccine-induced LETM should show marked clinical improvement after appropriate treatment.
KEYWORDS
COVID-19, transverse myelitis, mRNA COVID-19 vaccines, autoimmune disease
INTRODUCTION
Longitudinally extensive transverse myelitis (LETM) is a rare subtype of transverse myelitis (TM) which extends over three or more vertebral segments and presents with spinal cord dysfunction, including rapid onset weakness, sensory loss, and bowel and bladder dysfunction [1]. Central nervous system (CNS) demyelination disorder, systemic inflammatory disorders, infectious and paraneoplastic syndromes and vaccination are known causes of TM [2, 3]. Headache, dizziness, muscle pain and spasm, myalgia and paraesthesia are common neurological symptoms; however, rare cases of stroke, Guillain-Barre syndrome, facial palsy, seizures, acute disseminated encephalomyelitis and TM have been reported after COVID-19 vaccination [4]. We report the case of a 50-year-old man admitted to hospital 20 days after his second dose of the Pfizer COVID-19 vaccine with progressive lower extremity weakness and numbness, back pain and urinary retention. Investigation and imaging results were consistent with LETM.
CASE DESCRIPTION
AA 50-year-old man with no significant past medical history presented to the emergency department with progressive weakness and numbness of the bilateral lower extremities, back pain, and difficult urination for 2 days. He denied fever, chills, headache, dizziness, vision changes, chest pain, shortness of breath, abdominal pain, nausea and vomiting. He had no history of recent trauma, travel or contact with a sick person. He reported COVID-19 infection 10 months earlier with complete recovery without treatment. He had received his second dose of the Pfizer COVID-19 vaccine 20 days previously.
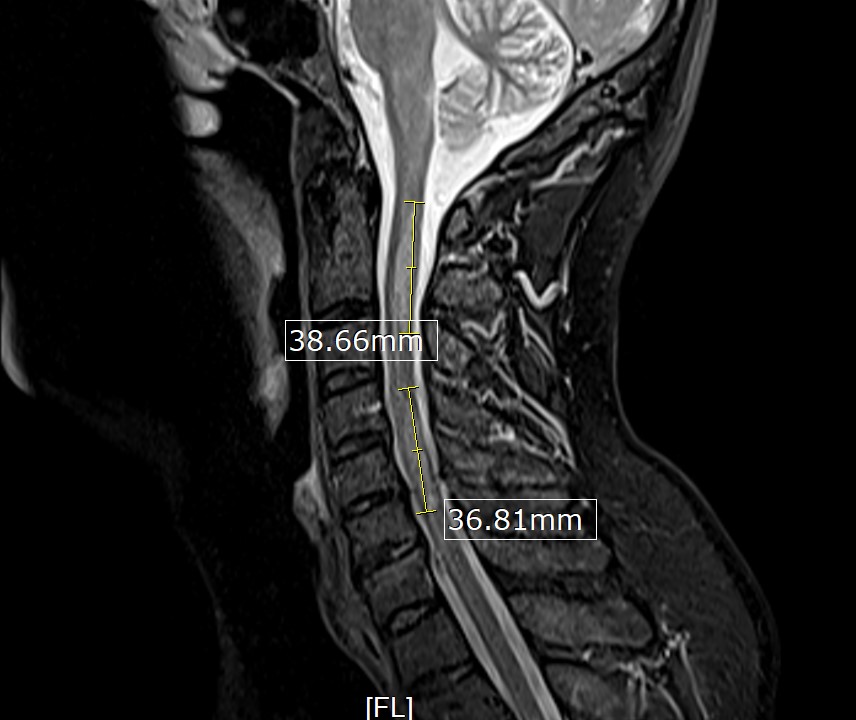
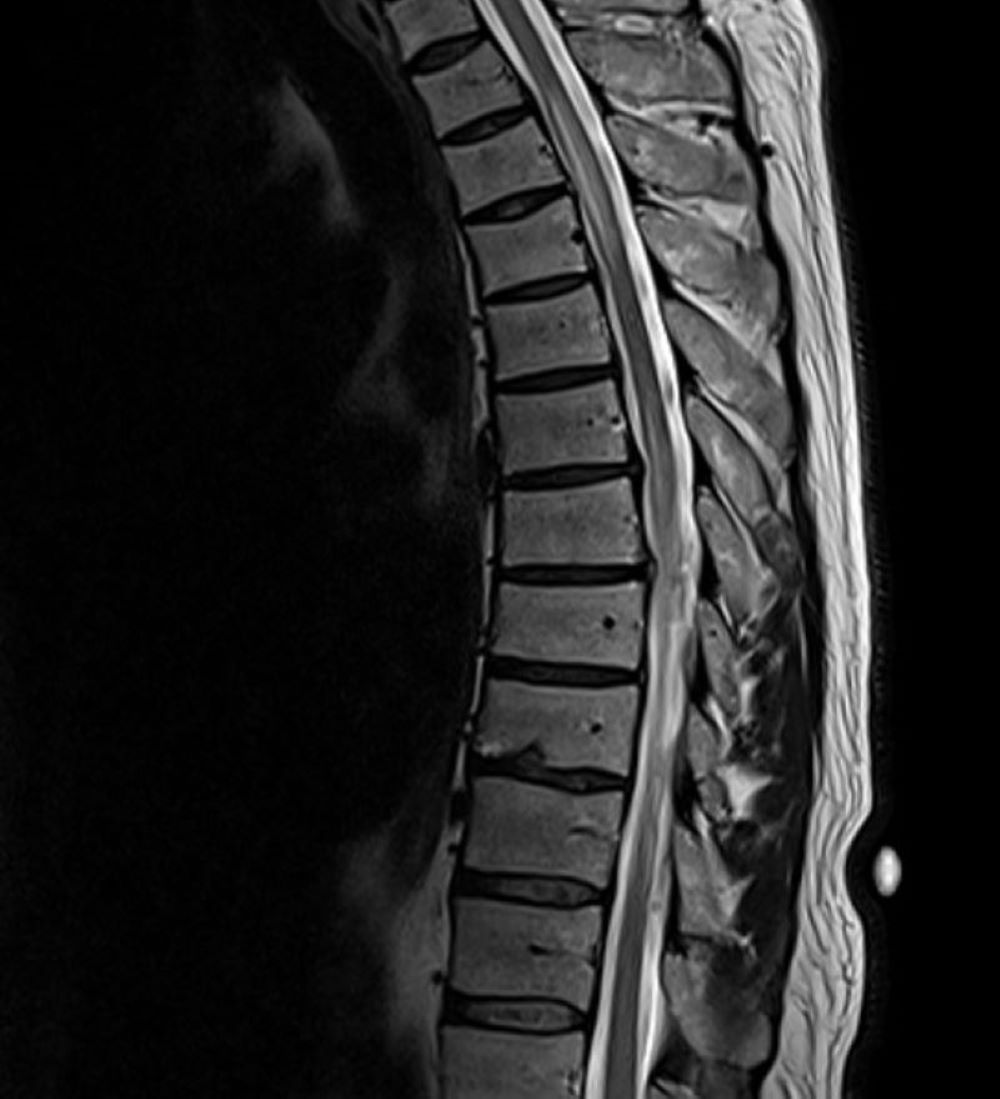
His initial vital signs were blood pressure of 136/72 mmHg, temperature of 36.7°C, heart rate of 76 beats/min, and respiratory rate of 16 breaths/min with 98% saturation on room air. The physical examination revealed decreased strength in the bilateral lower extremities (muscle power 4/5), decreased vibration sense and proprioception in the bilateral lower extremities with sensory level at T4, and brisk reflexes (3+) in all four extremities with a positive Babinski reflex and ataxic gait. The rest of the physical examination was unremarkable. The laboratory studies, including complete blood count and comprehensive metabolic panel, were unremarkable. A COVID-19 PCR test was negative. MRI of the brain was unremarkable. However, MRI of the spine (cervical/thoracic/lumbar) revealed multiple relatively long segments of increased cord signal diffusely throughout the cervical and thoracic cord with lesions mainly affecting C2–C5 (Fig. 1) and T2–T7 (Fig. 2).
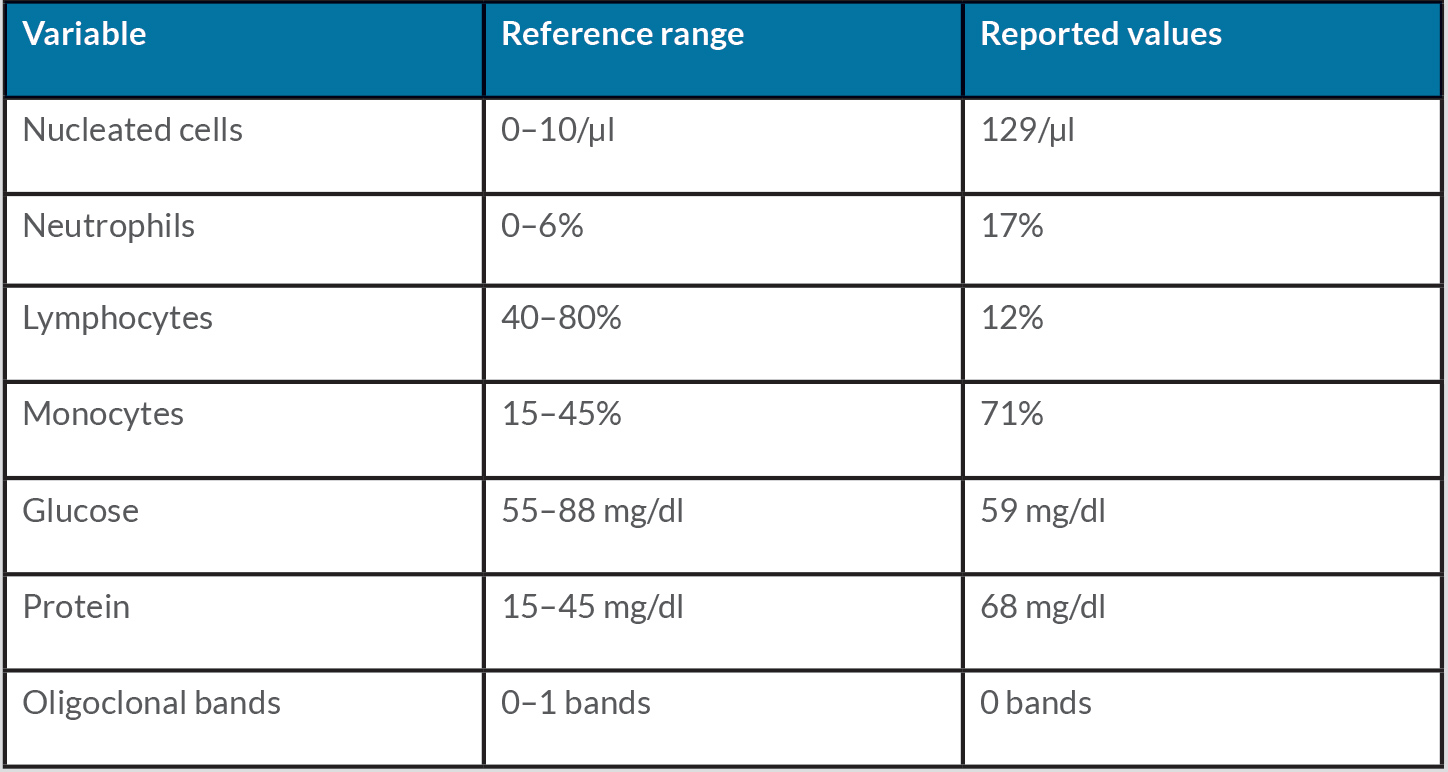
Cerebrospinal fluid (CSF) studies revealed pleocytosis and elevated protein with normal glucose, as shown in Table 1.
Figure 1. MRI of the cervical spine showing long segments of increased cord signal with C2–C5 involvement
Figure 2. MRI of the thoracic spine showing long segments of increased cord signal with T2–T7 involvement
Table 1. Cerebrospinal fluid study results during hospitalization
Additional work-up including CSF oligoclonal band, angiotensin-converting enzyme, aquaporin 4 receptor antibody (NMO-IgG), antimyelin oligodendrocyte glycoprotein (MOG) antibody, Lyme antibodies, meningitis panel, West Nile virus IgG and IgM, HIV, syphilis rapid plasma reagin, serum vitamin B12, serum copper, antinuclear antibody, antibody to an extractable nuclear antigen, rheumatoid factor and a paraneoplastic CSF panel, was unremarkable. The diagnosis of LETM secondary to COVID-19 vaccination was made by a diagnosis of exclusion. t
The patient was started on intravenous methylprednisone 1000 mg daily for 5 days. After five doses of methylprednisolone and physical therapy, his lower extremity weakness had improved (muscle power 5/5). He reported improved sensation in both feet, although numbness and tingling persisted. He was started on oral prednisone 1 mg/kg/day for 2 weeks, followed by a gradual taper, and discharged to the acute rehabilitation unit (ARU) to continue physical therapy. After 1 week of extensive physical therapy at ARU, he was discharged home. He continued outpatient physical therapy three times a week and completed recovery in about 1 month.
DISCUSSION
Acute TM is a rare, acquired neuro-immune spinal cord disorder with an incidence of 1.34–4.6 cases per million annually [5], and vaccine-associated myelitis is even more rare [6]. CNS demyelinating disorders (multiple sclerosis, neuromyelitis optica spectrum disorder (NMOSD), MOG antibody disorder, acute disseminated encephalomyelitis (ADEM)), systemic inflammatory disorders (systemic lupus erythematosus (SLE), sarcoidosis, Sjogren’s syndrome), infectious (enterovirus, HIV, syphilis) and paraneoplastic syndromes and vaccines (hepatitis B, measles, mumps, rubella, diphtheria, tetanus, pertussis) have been associated with TM [2, 3, 6]. TM has been reported as a complication of both COVID-19 infection and COVID-19 vaccination [7]. The pathogenesis of vaccine-associated myelitis is unclear. It is proposed that infectious agents and vaccine adjuvants may induce immune reactions by various proposed mechanisms such as molecular mimicry, epitope spreading, upregulation of cytokines, and polyclonal activation of B and T lymphocytes leading to TM [8]. However, mRNA COVID vaccines do not use adjuvants, and TM is less common with mRNA COVID vaccines than with viral vector COVID vaccines such as Johnson and Johnson and AstraZeneca [7]. mRNA COVID vaccines encode the pre-fusion SARS-CoV-2 spike protein; autoimmune reactions between SARS-CoV-2 spike protein antibody and tissue proteins or ACE2 receptors have been the proposed mechanism of mRNA COVID vaccine-induced TM [9]. t
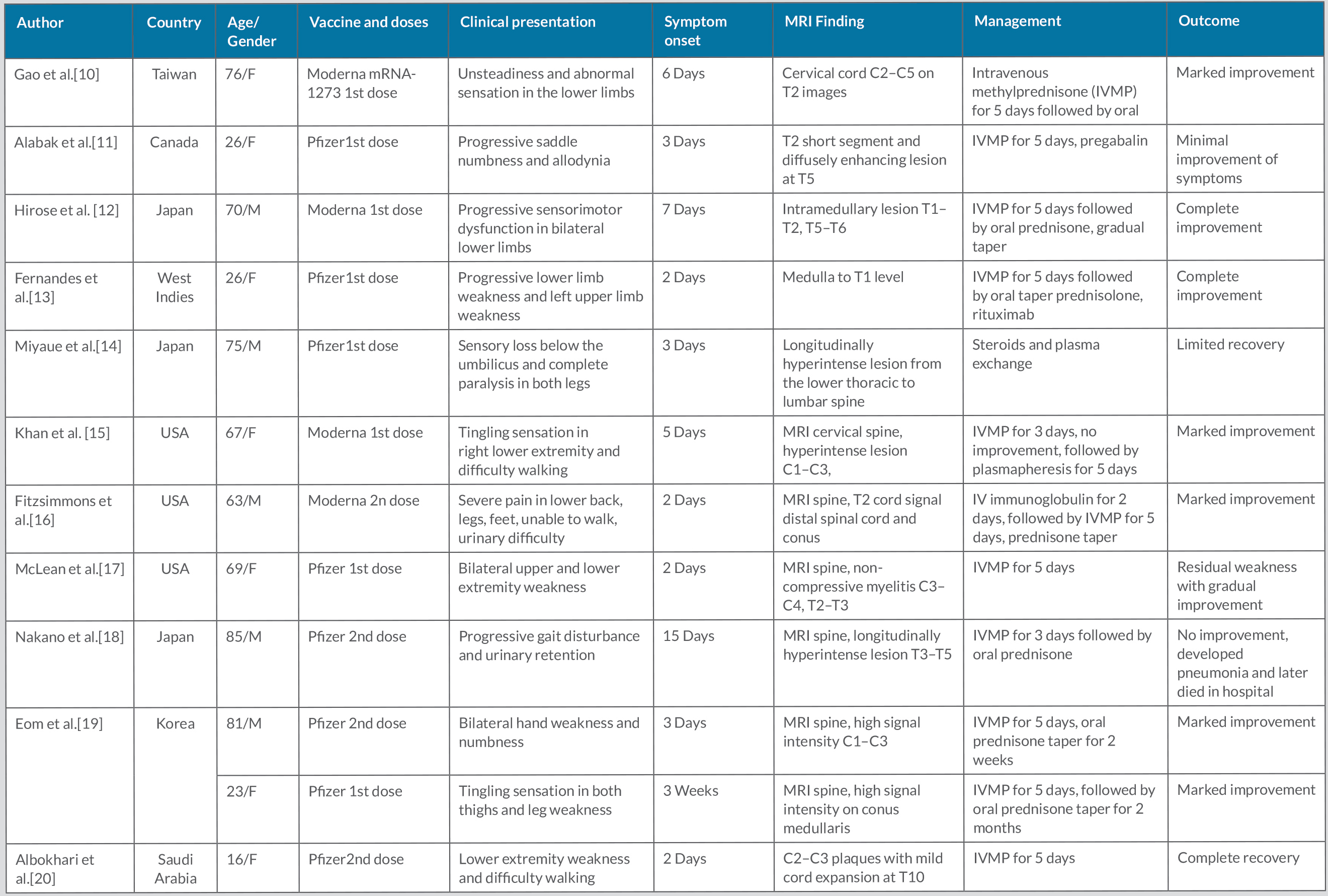
COVID-19 vaccination has played a significant role in curbing the COVID-19 pandemic. mRNA COVID vaccines are very safe. However, with mass immunization against COVID-19, various adverse events have been reported, including severe neurological adverse effects such as TM [4]. A literature review revealed 12 cases of TM after mRNA COVID-19 vaccination, which are summarized in Table 2. t
However, TM is a rare adverse event and marked clinical improvement can occur with prompt diagnosis and appropriate treatment, as shown in our case. Intravenous methylprednisone followed by oral prednisone is the most common therapeutic approach in reported cases of vaccination-induced TM. However, a few cases have been treated with subsequent plasma exchange and IV immunoglobulins along with methylprednisone therapy (Table 2). Further studies will be needed to determine the exact pathogenesis, risk factors, and treatment for mRNA COVID-19 post-vaccination TM.
Table 2. Cases of transverse myelitis after mRNA-based COVID-19 vaccination
CONCLUSION
This case highlights the rare association between LETM and mRNA COVID-19 vaccination and encourages clinicians to maintain a high index of suspicion for vaccine-induced TM for prompt diagnosis and treatment.