ABSTRACT
Eosinophilic myocarditis (EM) is a rare cause of acute heart failure. It can occur secondary to drug hypersensitivity, autoimmune diseases such as vasculitis, idiopathic hypereosinophilic syndrome (HES) or malignancy, but is often under-recognized and underdiagnosed, being confused with other causes of heart failure. While EM is associated with various clinical symptoms, it is rarely associated with cardiac tamponade that requires urgent pericardiocentesis. Here we describe a patient with EM who presented with cardiac tamponade and decompensated heart failure likely secondary to autoimmune disease.
LEARNING POINTS
- Work-up for hypereosinophilia should include the identification of treatable causes as well as end-organ dysfunction requiring urgent treatment.
- In patients presenting with acute heart failure and cardiac tamponade of unclear aetiology, eosinophilic myocarditis should be considered whether or not hypereosinophilia is present on presentation.
- When invoking the diagnosis of eosinophilic myocarditis, extensive efforts should be made to identify primary causes, such as autoimmune conditions including vasculitis.
KEYWORDS
Eosinophilic myocarditis, cardiac tamponade, eosinophilic granulomatosis with polyangiitis, immunoglobulin G4
INTRODUCTION
Eosinophilic myocarditis (EM) is a rare and life-threatening form of cardiomyopathy. It is associated with various aetiologies, including autoimmune conditions such as eosinophilic granulomatosis with polyangiitis (EGPA) and systemic lupus erythematosus, idiopathic hypereosinophilic syndrome (HES), infections, malignancy and clozapine overdose [1, 2]. EM can be difficult to recognize clinically because of its varying manifestations, ranging from vague dyspnoea to acute heart failure [1]. Here, we describe a case of EM with a presentation of acute decompensated heart failure and cardiac tamponade.
CASE DESCRIPTION
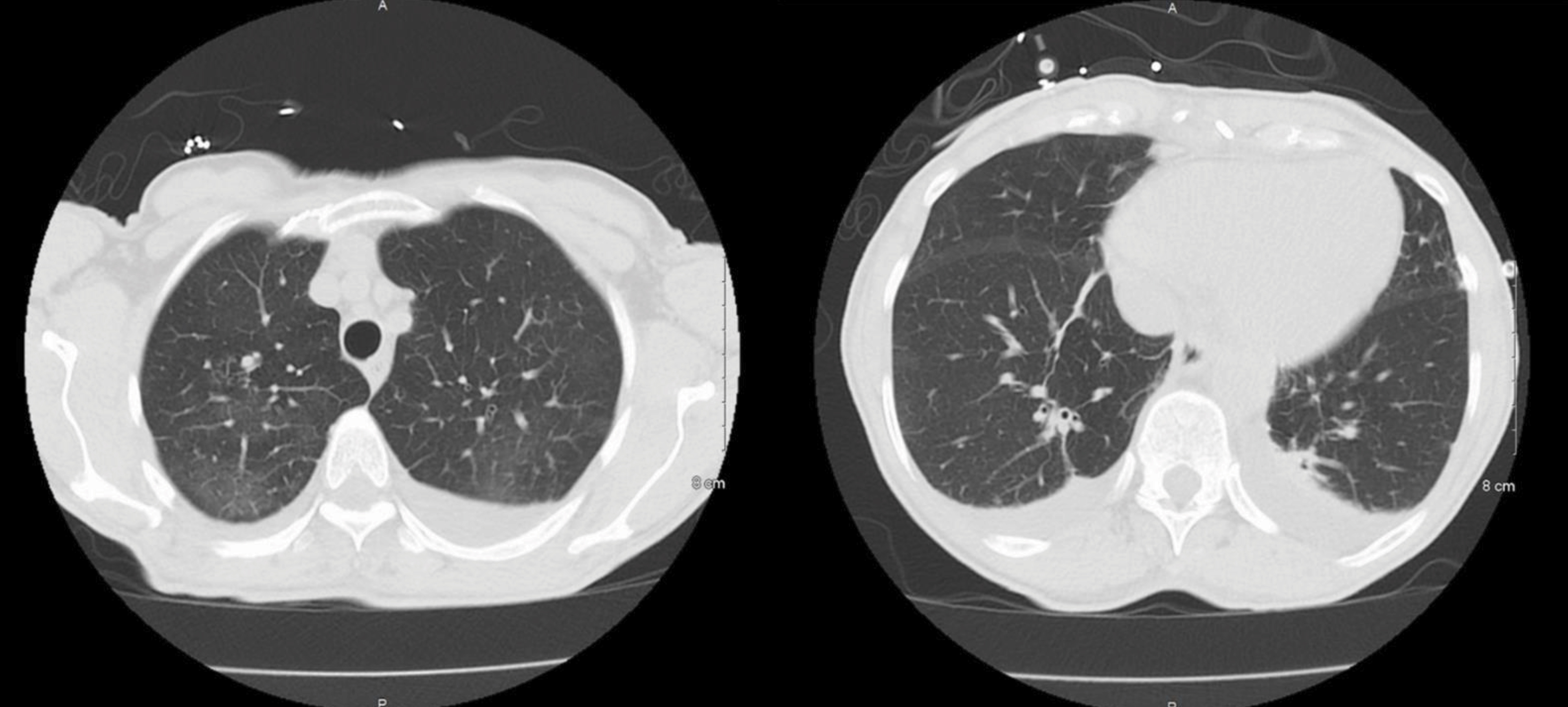
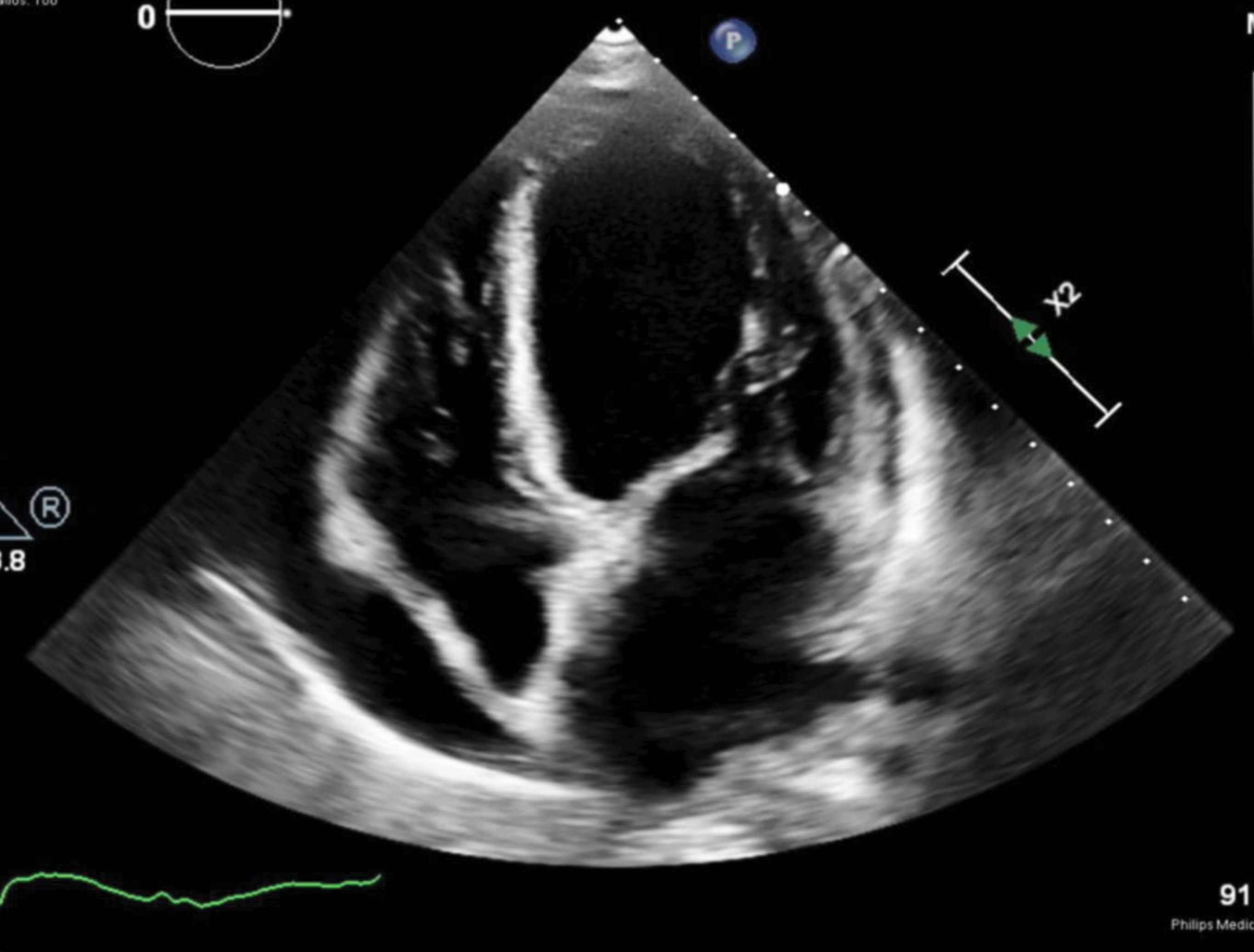
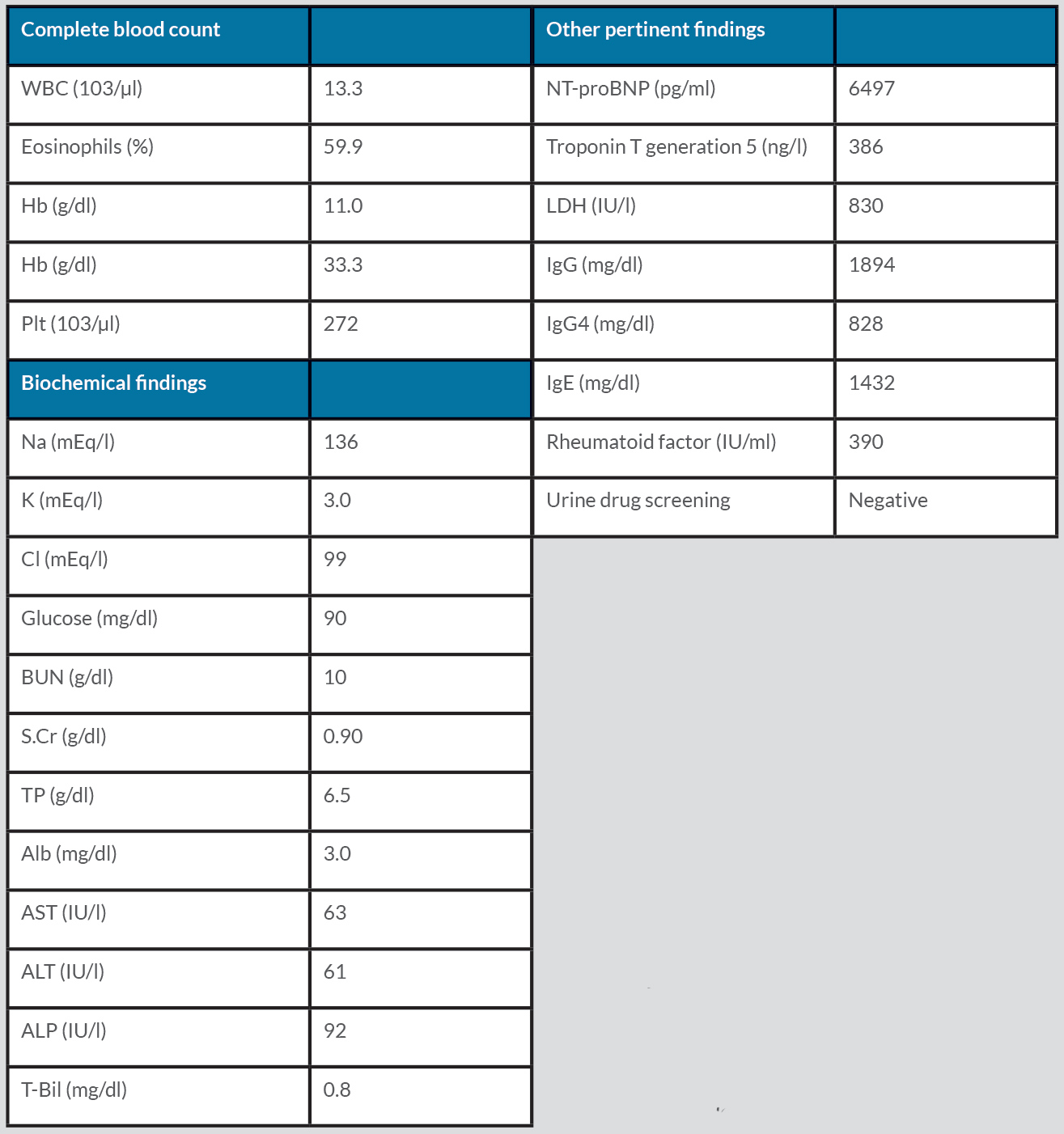
A 73-year-old woman with an unspecified chronic cough presented with progressive exertional dyspnoea for 4 weeks. She also reported orthopnoea, paroxysmal nocturnal dyspnoea, bilateral lower extremity oedema, and an unintentional weight loss of 2.72 kg over the past 2 months. She was on no medications or supplements. She denied smoking, vaping or recreational drug use. On physical examination, vital signs were all within normal limits. Notable findings included jugular venous distention to the mid-neck, bibasilar crackles, and 2+ pitting oedema of the lower extremities. Initial laboratory tests were significant for leucocytosis with eosinophilia, elevated troponin, and elevated NT-proBNP (Table 1). ECG showed low QRS voltage but no ST-T changes. Chest computed tomography (CT) showed scattered ground-glass opacities bilaterally with a mild pleural effusion (Fig. 1), while transthoracic echocardiography (TTE) showed a left ventricular ejection fraction (LVEF) of 35–40%, global hypokinesia with a moderate–large pericardial effusion, and inspiratory right ventricular collapse concerning for acute decompensated heart failure and cardiac tamponade (Fig. 2). A pericardiocentesis was performed, which was significant for elevated white blood cells (758/µl, neutrophils 53%, lymphocytes 46%). The culture and cytology of pericardial effusion came back negative.
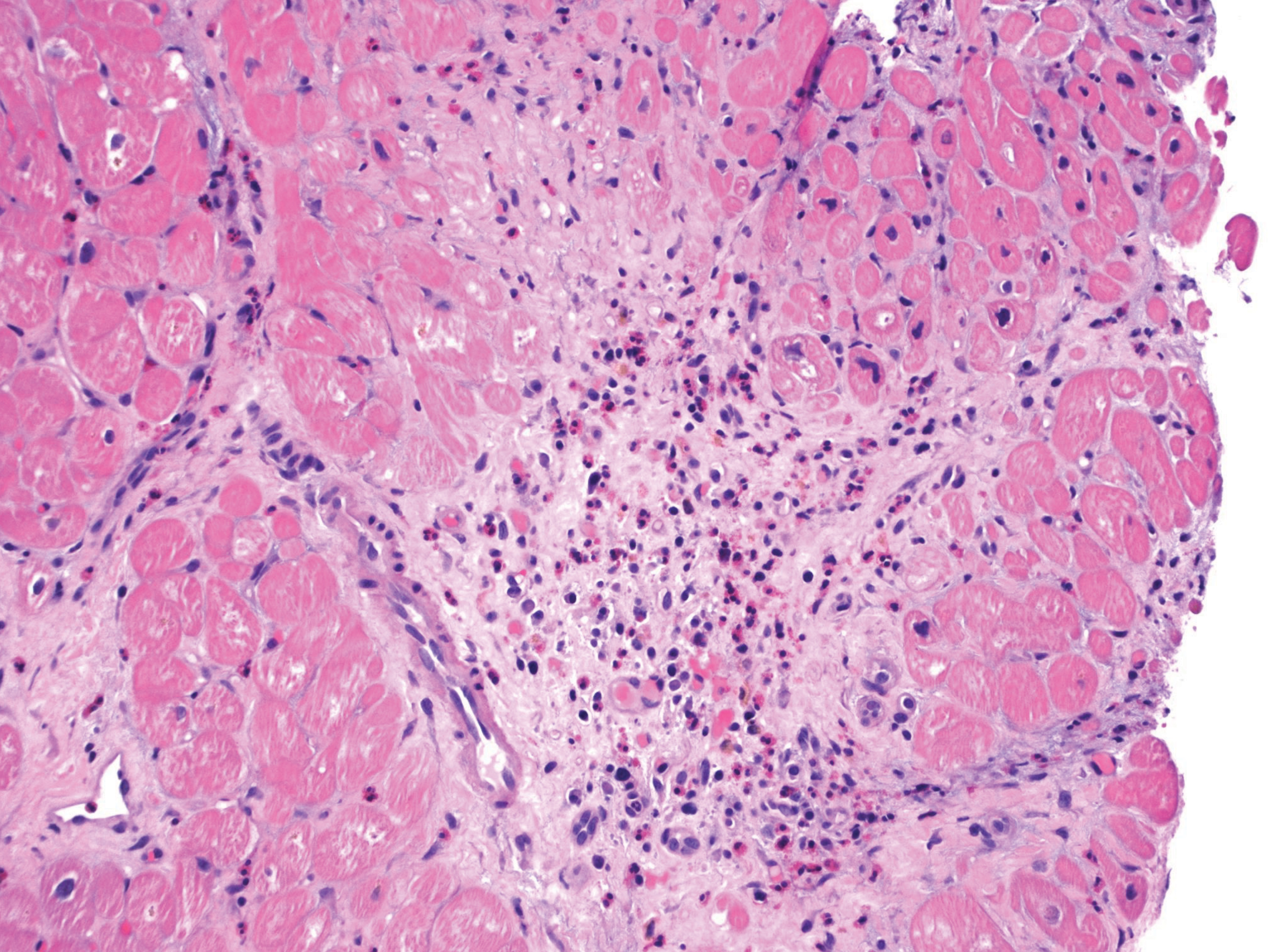
Due to concerns for myocarditis as the underlying cause of the acute heart failure and tamponade, an endomyocardial biopsy was performed. It showed significant infiltration of eosinophils with fibrotic changes (Fig. 3). Further laboratory testing was ordered to elucidate the cause of the eosinophilia, which is summarized in Table 1. Positive laboratory findings included elevated immunoglobulin G (IgG) (1894 mg/dl), IgE (1432 mg/dl), IgG4 (828 mg/dl) and rheumatoid factor (390 IU/ml). Antineutrophil cytoplasmic antibodies (ANCA) came back negative. During her hospital stay, the patient was given high-dose IV methylprednisolone, 1 g/day, for 3 days and subsequently transitioned to a tapering regimen starting at 40 mg prednisone daily, decreasing by 5 mg every week. A repeat TTE on hospital day 10 showed a recovering LVEF of 45–55%. Over time, her symptoms improved, and the peripheral eosinophilia resolved. She was discharged with close outpatient follow-up with cardiology and rheumatology.
Figure 1. Computed tomography of the chest on admission showed scattered ground-glass opacities bilaterally with mild pleural effusion
Figure 2. Transthoracic echocardiogram on admission showed a left ventricular ejection fraction of 35–40%, global hypokinesia with a moderate–large pericardiac effusion, and inspiratory right ventricular collapse concerning for cardiac tamponade
Figure 3. CT abdomen obtained 12 days after admission showing a complex, thick-walled gas and fluid collection in the lower right abdomen measuring 12.5 x 7.2 x 7.5 cm
Table 1. Main laboratory data.
Alb, albumin; ALP, alkaline phosphatase; ALT, alanine aminotransferase; AST, aspartate aminotransferase; Ca, calcium; Cl; chloride; Hb, haemoglobin; Hct, haematocrit; Ig, immunoglobulin; K, potassium; LDH, lactate dehydrogenase; Na, sodium; NT-proBNP, N-terminal-pro brain natriuretic peptide; Plt, platelets; S.Cr, serum creatinine; T-Bil, total bilirubin; TP, total protein; WBC, white blood cells.
DISCUSSION
We have described a case of EM complicated by pericardial effusion and cardiac tamponade. In patients with peripheral hypereosinophilia, the symptomatic presentation is varies widely. Thus, while pursuing primary causes, clinicians must also remain wary of potential end-organ dysfunction, as it can become irreversible and requires urgent intervention.
Eosinophilia is defined as a peripheral eosinophil count >500/µl, while hypereosinophilia is defined as a count >1500/µl. This distinction is made because hypereosinophilia is associated with a higher risk of end-organ damage. Peripheral hypereosinophilia associated with end-organ damage is termed HES. However, hypereosinophilia is not always evident early in the disease process, even in patients exhibiting organ dysfunction. It is estimated that peripheral eosinophilia is absent in up to 25% of patients [1]. HES most often involves the skin at initial presentation, manifesting as rash or angioedema, but more serious complications of HES include eosinophil-mediated myocardial injury, respiratory failure and severe neuropathy [3]. Cardiac involvement at initial presentation is rare, yet it remains a major cause of morbidity and mortality in these patients [3]. Even less common is myocardial involvement presenting as tamponade, as seen in the patient described above. One study suggested that cardiac tamponade was seen at presentation in a mere 6% of patients with histologically proven EM [1]. While most patients with EM present with dyspnoea and/or chest pain, the disease can be fatal in its fulminant form. Thus, clinicians need to recognize EM as a differential diagnosis for those with acute heart failure or cardiac tamponade due to unknown causes.
While hypereosinophilia has broad differential diagnoses, mnemonics such as CHINA (Connective tissue disease, Helminths or parasites, Idiopathic, Neoplasm, Allergy) or ALLERGIC (Adrenal insufficiency, Lymphoma, L-tryptophan deficiency, Eczema, Respiratory causes, Gastroenteritis, Infections, Collagen vascular disease) cover most of them and are valuable in ensuring a complete work-up. While the primary cause of EM is yet to be identified in our patient, given her history of unspecified chronic cough and elevated immunoglobulins, ANCA-negative EGPA or immunoglobulin IgG4-related disease is high on the differential diagnosis. EGPA, previously known as Churg-Strauss syndrome, is one of the critical causes of hypereosinophilia. Asthma occurs in more than 90% of patients with EGPA, and studies found that patients with EGPA were diagnosed with asthma 3–9 years before EGPA diagnosis [4]. Interestingly, ANCA is positive in only 30–40% of those with EGPA, and studies suggest that their ANCA status may portend differing disease manifestations. Compared with ANCA-positive cases, ANCA-negative EGPA is associated with a higher prevalence of cardiac or pulmonary complications [5]. Thus, it is crucial to recognize that a negative ANCA does not exclude EGPA.
Evidence is scarce regarding treatment for EM. While a systematic review published in 2017 suggested that corticosteroids might improve the survival of EM patients, there is no evidence or guidelines to date on how we should dose or taper corticosteroids or on the superiority of other immunosuppressants in EM. In this case, due to concern for significant cardiac injury, high-dose corticosteroids were initiated after endomyocardial biopsy. Close outpatient rheumatology follow-up is warranted for those with EM due to unspecified causes, given the high likelihood of autoimmune disease such as ANCA-negative EGPA as the underlying pathogenesis.
.