ABSTRACT
Ofatumumab is a monoclonal antibody used in the treatment of recurrent and progressive chronic lymphocytic leukaemia (CLL) and was recently approved for the treatment of multiple sclerosis.
We describe the case of a 68-year-old man who presented with complaints of irregular pulse readings while undergoing ofatumumab treatment for recurrent CLL. Electrocardiograms (ECGs) demonstrated premature ventricular contractions (PVCs) which eventually caused cardiomyopathy and failed to resolve despite ablative therapy. Ofatumumab-induced PVCs are confirmed in this case by the existence of documented PVCs on ECGs and the disappearance of these PVCs after the completion of ofatumumab treatment.
To the best of our knowledge, there have been no previously reported cases of PVCs associated with ofatumumab in the literature.
LEARNING POINTS
- Ofatumumab is a potential cause of arrhythmias and should be taken with care, particularly in individuals with underlying cardiac disease.
- Work-up of arrhythmias should include a comprehensive medication review as they can be caused by medications such as ofatumumab.
KEYWORDS
Premature ventricular contraction, ofatumumab, chronic lymphocytic leukemia
INTRODUCTION
Ofatumumab is an anti-CD20 monoclonal antibody with a predilection for B-cells. It has been shown to be effective in treating patients with recurrent or progressive chronic lymphocytic leukaemia (CLL), particularly those in complete or partial remission after two or more lines of prior therapy [1].
Some reported adverse effects of ofatumumab include neutropenia, infections, thrombocytopenia, dyspnoea, infusion reactions, fever, nausea and fatigue, with little mention of cardiac abnormalities. Herein, we report the case of a patient with recurrent CLL who developed new-onset premature ventricular contractions (PVCs) leading to cardiomyopathy while on ofatumumab.
CASE DESCRIPTION
A 68-year-old man undergoing treatment for recurrent CLL presented to the oncology urgent care clinic with complaints of irregular pulse readings. He had a past medical history of CLL, autoimmune haemolytic anaemia, basal cell carcinoma, hereditary haemochromatosis (HH) and hypertension. Eleven years before this presentation, he had been diagnosed with CLL and received six cycles of rituximab and bendamustine treatment, which was successful.
Eight years after completing treatment for the initial diagnosis of CLL, and 4 months before this new presentation to urgent care, a computerized tomography scan revealed a mildly enlarged mesenteric lymph node. Subsequent bone marrow biopsy confirmed CLL recurrence for which the patient was initiated on a treatment plan consisting of five initial cycles of ofatumumab, followed by a maintenance plan of 12 cycles of ofatumumab to be administered every 8 weeks for 2 years.
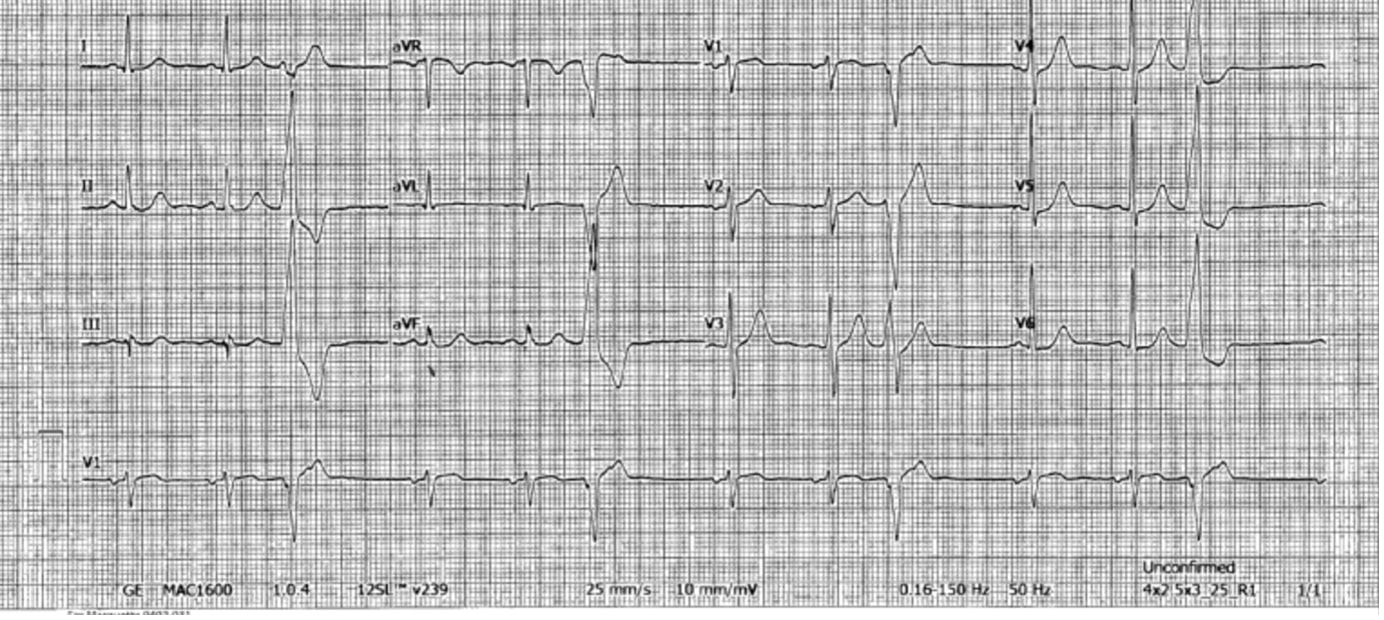
At the time of presentation, the patient was in between the second and third cycles of the initial five-cycle ofatumumab treatment. He denied chest pain, shortness of breath, dyspnoea, dizziness, light-headedness or syncope, and had a normal cardiovascular examination. His laboratory values were within normal limits, but a 12-lead electrocardiogram (ECG) showed sinus rhythm with frequent PVCs (Fig. 1).
Figure 1. Urgent care 12-lead electrocardiogram showing premature ventricular complexes
The patient was referred to the Cardio-Oncology (C-O) clinic for further analysis and care of the PVCs. At the C-O visit, 1 month after the urgent care visit, he reported he had heart fluttering sensations which began after commencing ofatumumab. The sensations began after each ofatumumab infusion and usually resolved in a few days. His symptoms improved with a reduction in the frequency of ofatumumab infusions. He reported a significant intake of caffeine before the onset of symptoms; however, he continued to have frequent PVCs on ambulatory ECGs even after he terminated intake of caffeinated drinks. He denied intake of significant sugars or alcohol, and his haemoglobin, thyroid stimulating hormone, magnesium, potassium and calcium levels were within normal limits. A transthoracic echocardiogram (TTE) revealed a normal left ventricular ejection fraction (LVEF) of 55–60%, while cardiac magnetic resonance (CMR) revealed non-specific fibrosis in the basal anteroseptum/aortic annulus; however, no ischaemia or infarction was noted.
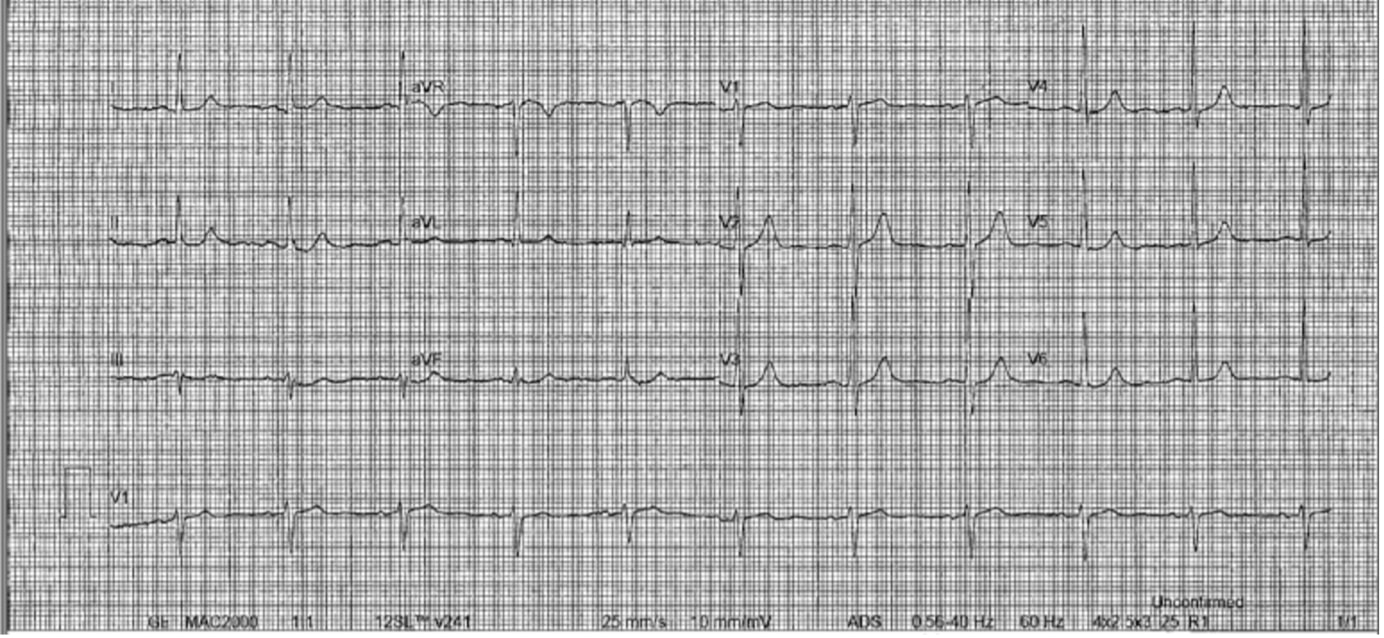
The patient was placed on a cardiac ambulatory telemetry monitor which revealed a large burden of ventricular ectopy (~21%) with many non-sustained ventricular tachycardia runs and bigeminy typically occurring during waking hours. He was initiated on metoprolol which improved his symptoms, but the PVCs remained present on ECGs throughout ofatumumab cancer therapy. One and a half years after the urgent care visit, a TTE revealed dilated left and right ventricular cavities, and a decreased LVEF of 40–45%. Due to the symptoms, new onset cardiomyopathy, prior cardiac MRI findings, and the need to continue ofatumumab treatment, he was referred to Electrophysiology and a PVC ablation was performed. However, the large burden of PVCs persisted on subsequent ECGs and resolved only after completion of maintenance ofatumumab treatment 2.5 years after the initial dose. This was confirmed on ambulatory telemetry monitoring (<1% PVC burden over a 24-day monitoring period) obtained 2 months after ofatumumab treatment completion (Fig. 2). An echocardiogram performed after completion of the ofatumumab treatment demonstrated a normalized LVEF of 55–60% and normalized left ventricle cavity size. He has since been placed on acalabrutinib (a newer anti-CD20 monoclonal antibody), with no adverse cardiac effects noted.
Figure 2. Electrocardiogram 2 months after the completion of ofatumumab chemotherapy, revealing a normal sinus rhythm and no rhythm abnormalities
DISCUSSION
While antineoplastic agents have well-documented cardiovascular complications, the known cardiac adverse effects of ofatumumab are sparse [2]. A few clinical trials have assessed ECG findings before and after ofatumumab administration and showed no significant relationship between ofatumumab and cardiac repolarization, and no reported cases of PVCs [3].
In the absence of other possible causes of PVCs, and PVC onset/cessation correlating with the drug, this case represents ventricular arrhythmias caused by ofatumumab. Other antineoplastic drugs such as paclitaxel have demonstrated PVCs as known adverse events among other cardiac side effects. Furthermore, there have been reports of ifosfamide-associated PVCs [4], and PVCs have also been described with the use of rituximab monoclonal antibody treatment [5].
The exact mechanism of ofatumumab-induced PVCs as in this case is unclear. It is important to note the history of HH in this patient which could have predisposed him to arrhythmias, although no cardiac involvement of the HH was seen on CMR or TTE. Also, the PVCs would have been unlikely to reverse without treatment targeted at HH.
Ofatumumab was recently approved by the FDA for the treatment of multiple sclerosis [6]. As such, for patient comfort and safety, further studies of ofatumumab precisely focused on its cardiac side effects are necessary. In our case, the patient felt discomfort due to palpitations from the PVCs while already undergoing mental and physical stress from antineoplastic treatment. The PVCs also caused cardiomyopathy, so heart failure could have been a consequence.
To the best of our knowledge, this is the first reported case of ofatumumab-induced PVCs. Although ofatumumab is a relatively safe and effective option in the management of CLL, much remains unknown about the cardiac side effects of this drug recently approved by the FDA for the treatment of multiple sclerosis. The temporal relationship between ofatumumab therapy and the patient-reported palpitations, with confirmed PVCs on the ECG and telemetry, suggests that the arrhythmia was ofatumumab-induced. This could have been a case of cardiac hypersensitivity to the drug; however potential cardiotoxic arrhythmogenic effects need to be considered. Therefore, treating physicians should be aware of the potential arrhythmogenic effect(s) of ofatumumab and be especially cautious with patients who have underlying cardiac diseases.