ABSTRACT
Vitamin B12 deficiency is a common finding in medical practice. It is easily treated with supplementation and typically has a favourable prognosis. In rare circumstances, it can hide a severe disease that should be promptly addressed. We report the case of an acute myeloid leukaemia presenting as an initially predictable B12 deficiency in a vegetarian patient with chronic gastritis. The supplementation rapidly corrected the deficit and the accompanying cytopenias. However, in the following month the cell counts fell once again, leading to the suspicion that other aetiology could be lying beneath the surface. Maintaining a normal peripheral blood smear, the bone marrow biopsy showed myeloblasts and extensive fibrosis compatible with the diagnosis of acute myeloid leukaemia. The neoplasm justified the vitamin deficit by excessive cellular turnover, a vicious cycle only uncovered after supplementation and that ultimately led to the patient’s death.
LEARNING POINTS
- Vitamin B12 is a common aetiology of pancytopenia and is usually caused by gastric malabsorption.
- When supplementation does not correct the haematological deficit, central causes must be considered. Acute myeloid leukaemia is one possibility, but causes peripheral blood smear abnormalities in almost all patients.Diagnosis should include lumbar puncture and a thorough search for the aetiology; treatment is directed towards the aetiology.
- Neoplastic diseases should be always excluded when correction of the deficit does not resolve cytopenias.
KEYWORDS
Acute myeloid leukaemia, pancytopenia, B12 deficiency
INTRODUCTION
Vitamin B12 deficiency is a ubiquitous diagnosis in the developed world, highly associated with vegetarian regimens. Other common causes exist, such as infections and gastrointestinal disease. If severe, it can cause disorders that go from a mild isolated megaloblastic anaemia to a severe pancytopenia [1]. These complications occur because of the impaired mitosis of which B12 vitamin is a cofactor [2]. In rare cases, the deficit can be observed in the context of excessive cell consumption due to high cellular turnover, as happens with neoplasms [3]. We present a unique case of pancytopenia seemingly induced by B12 vitamin deficiency – and initially corrected by supplementation – that uncovered a hidden acute myeloid leukaemia only apparent after bone marrow biopsy.
CASE DESCRIPTION
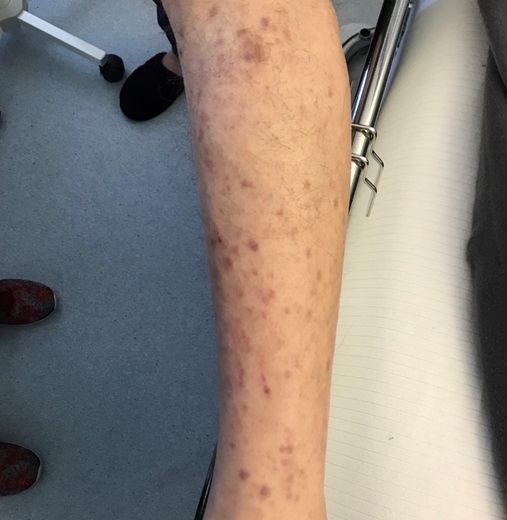
We present the case of a 70-year-old male with chronic gastritis and diabetes mellitus, controlled with metformin. In the previous six months he had adopted a vegan-based diet without consumption of any animal products. Three months later, he started complaining of asthenia, denying any other complaints, including blood loss, fever or anorexia. On admission, he had an excellent global status, albeit some mucocutaneous pallor. A complete blood count (CBC) showed severe anaemia with a haemoglobin level of 6.4 g/dL, with a mean cell volume of 104 fL and a mean haemoglobin level of 36 pg. He also had a mild leukopenia of 3.92x109 leukocytes per litre, with 50% neutrophils and 46% lymphocytes with no immature cells, and a thrombocytopenia of 59x109 platelets per litre. At this point, we had a pancytopenia of unknown aetiology. The peripheral blood smear (PBS) was normal. The reticulocyte index was low, indirect bilirubin was high, as was his lactate dehydrogenase (LDH), and haptoglobin was low. All these parameters pointed to the occurrence of haemolysis. Iron parameters were normal, as well as folic acid concentration; however, B12 vitamin was undetectable. The abdominal ultrasound excluded hepatosplenomegaly or lymphadenopathies. An upper endoscopy was performed, showing mild gastritis, and anti-parietal cell antibodies were positive. The patient was then diagnosed with pancytopenia caused by severe B12 vitamin deficiency. The abnormal giant erythrocytes were being destroyed peripherally, thus justifying the elevation of haemolytic parameters. The vitamin deficit was multifactorial – he was on a vegan diet, he was medicated with metformin and had an autoimmune gastritis, both of which were reducing vitamin B12 absorption. He was started on high dose intravenous B12 supplementation and had normalisation of bilirubin and LDH. The haemoglobin, leukocyte and platelet levels slowly increased accordingly to the respective half-lives in circulation. One month later, he complained of relapsing asthenia and new onset cutaneous lesions in both legs (Fig. 1).
Figure 1. Infiltrates of leukaemic cells, known as leukaemia cutis, in the patient’s legs.
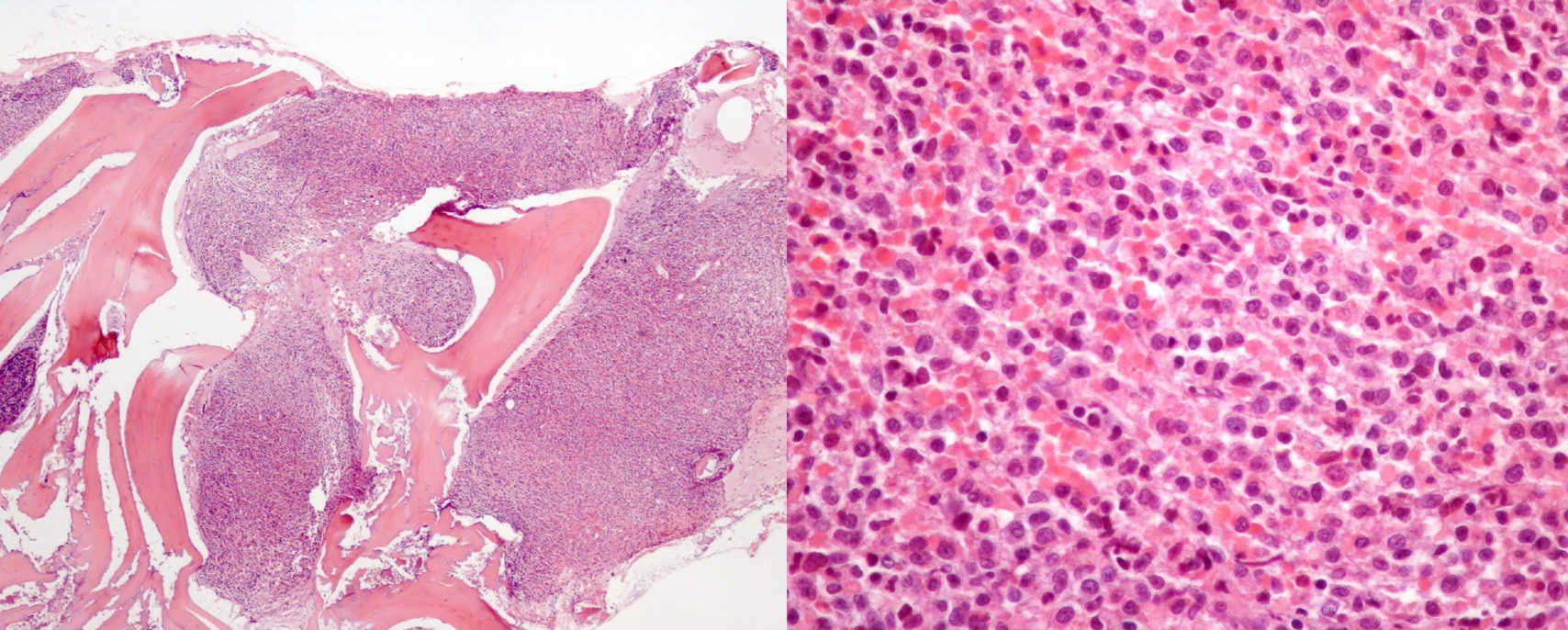
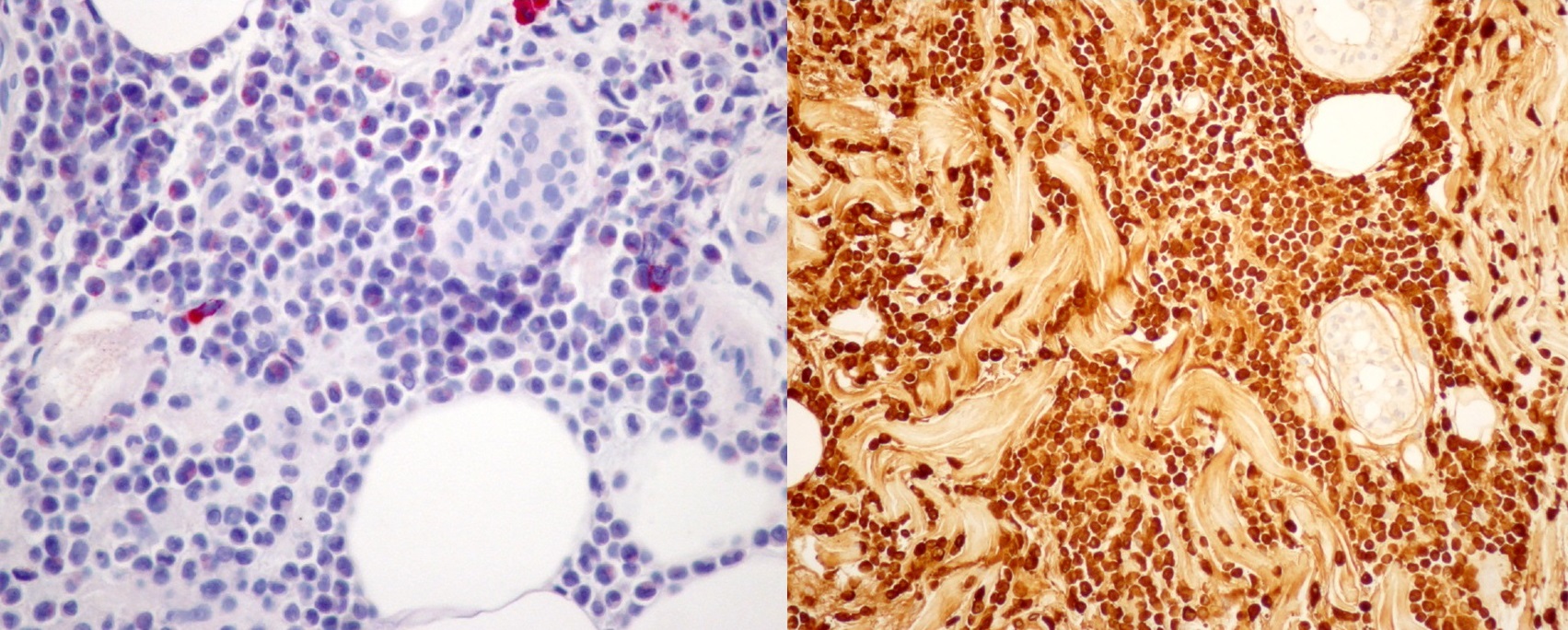
CBC showed a relapse of the pancytopenia, with no immature cells and a normal PBS. Indirect bilirubin and LDH were once again elevated. B12 levels were >2000 pg/mL (for a normal cut-off of 187–883 pg/mL). In short, we had a patient with persistent pancytopenia and signs of peripheral haemolysis despite normal levels of B12 vitamin, previously in deficit. The differential diagnosis now included central/bone marrow and peripheral causes of pancytopenia. From the peripheral causes, splenomegaly and intravascular disseminated coagulopathy were excluded with a thorough clinical history and abdominal ultrasound. Bone marrow secondary causes could include autoimmune, infectious and toxic aetiologies that were excluded since all autoimmune studies, cultures and serologies were negative. In terms of potential toxicity, there was no history of alcoholism, intravenous drug consumption or other medications or supplements not listed. A full body computed tomography scan was performed and was normal. The serum calcium and protein electrophoresis were normal. A cytological approach to the bone marrow was tried unsuccessfully due to dry tap. A bone marrow biopsy was then performed, showing a hypercellular marrow with extensive neoplastic invasion and fibrosis (Fig. 2), compatible with acute myeloid leukaemia with monocytic differentiation (AML-MD). A biopsy of the maculopapular lesions was also performed, showing monocytic neoplastic infiltration of the dermis and hypodermis (Fig. 3) – the rare phenomenon of leukaemia cutis – corroborating the diagnosis of AML.
Figure 2. A section of the bone marrow biopsy performed, showing a high percentage of immature cells (haematoxylin and eosin [H&E] stain), on the left with 40x magnification and on the right with 200x magnification).
Figure 3. A section of the skin biopsy, confirming the diagnosis of acute myeloid leukaemia by demonstrating the presence of blasts in the dermis. (on the left, leder stain with 40x magnification, on the right lysozyme stain with 200x magnification).
DISCUSSION
Vitamin B12 acts as a cofactor in the folate metabolism, being crucial for cell division. A deficit in B12 creates a relative deficit in folate and impairs cell replication, affecting mostly tissues that are highly dependent on replication, such as haematopoietic cells. The deficit can occur in patients with dietary regimens deprived of products of animal origin, chronic gastritis and prescription of some over-the-counter drugs, such as metformin. All three impair the absorption of the vitamin in the gastrointestinal tract [1,4]. In these cases, the supplementation is enough to return the cell counts back to normal. In the first part of our case, a severe B12 vitamin deficit was noted and attributed to all three causes listed above. The fact that the cell counts and symptoms all ameliorated significantly with the vitamin supplementation gave strength to the diagnosis. However, the second part of our case brings us back to square one – the cytopenias returned under B12 vitamin supplementation and there was a new symptom: a maculopapular rash on the legs. This refractoriness should redirect the differential diagnosis to other, more uncommon aetiologies of pancytopenia. Everything that can potentially hinder haematopoiesis should be excluded, such as infections, drugs, toxins and autoimmunity. When every secondary cause is excluded, thinking should be directed towards central causes of inadequate haematopoiesis, such as acute or chronic leukaemia, multiple myeloma, myelodysplastic syndrome, myelofibrosis or metastasis. All these aetiologies should be excluded via bone marrow biopsy. In the present case, the bone marrow aspirate was inconclusive due to the so-called dry tap, which is an important finding by itself, meaning that there is extensive fibrosis that precludes the aspiration. The biopsy confirmed the presence of an AML-MD, which the skin biopsy confirmed by showing dermal leukaemia cutis, which is an infrequent phenomenon [5]. It is even more unusual for a patient with AML not to have circulating myeloblasts at the time of diagnosis; it has also been demonstrated especially in acute promyelocytic leukaemia[6,7]. Some cases are described where skin presentation precedes the appearance of immature cells in the blood [8]. B12 deficiency can, ultimately, be explained by consumption from the leukaemic cells, in avid proliferation. The deficit, on the other side, exacerbated the cytopenias already caused by marrow infiltration, creating a vicious cycle that was partially relieved with supplementation. To the best of our knowledge there are only a handful of cases of leukaemia presenting concurrently with B12 deficiency or mimicking cell changes typically attributed to AML. Ordinarily, patients have changes in PBS which promptly suggests the diagnosis [9–11]. In this case, not only did the patient have none of these changes but also the pancytopenia resolved with B12 supplementation.
CONCLUSIONS
B12 deficiency is common diagnosis, even more in patients with multiple risk factors. However, an appropriately lasting follow-up can reveal a relapse of the deficit and its manifestations, which happened in our case. This allowed us to think beyond a simple vitamin deficit and reach the final diagnosis of AML. This case emphasises the importance of never forgetting unusual presentations of certain illnesses, even when other causes seem apparent.