ABSTRACT
Treatment strategies for patients with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) continue to be heavily researched and ever-changing. Recent data has suggested that combination therapy with dexamethasone, remdesivir and baricitinib could decrease the severity and length of illness in patients with severe SARS-CoV-2. However; the data regarding the safety and side effects related to this combination therapy are limited to case reports. The purpose of this case report is to highlight a potentially life-threatening side effect of one or all medications mentioned above.
LEARNING POINTS
- Current National Institutes of Health treatment guidelines recommend remdesivir for patients with a high risk of progression. In patients requiring minimal supplemental oxygen, remdesivir or dexamethasone monotherapy is recommended, while in patients requiring high-flow oxygen or non-invasive ventilation, dexamethasone monotherapy or dexamethasone plus remdesivir is recommended. Baricitinib or tocilizumab can be added in patients requiring oxygen supplementation.
- Clinicians should be aware of transient leukocytopenia that can be induced with combination therapy of dexamethasone, remdesivir and baricitinib during the early phase of treatment of SARS-CoV-2 patients.
- The evaluation approach for leukopenia should consider autoimmune disorders, inflammatory diseases, infections, malignancy, and medication and toxin exposure.
KEYWORDS
SAR-CoV-2 pneumonia, transient leukopenia, remdesivir, baricitinib, dexamethasone
INTRODUCTION
SARS-CoV-2 patients experience a wide variety of clinical symptoms ranging from asymptomatic to severe illness, guiding therapeutic management. The Adaptive COVID-19 Treatment Trial (ACTT-1) is a randomized, double-blind, placebo-controlled trial which demonstrated that a 10-day course of intravenous (IV) remdesivir in severe SAR-CoV-2 patients (SpO2 <94% on room air at sea level, mechanical ventilation (MV) or extracorporeal membrane oxygenation (ECMO)) shortened time of recovery compared with placebo [1]. The second stage of ACTT-2 evaluated the outcomes of combination therapy using a 10-day course of remdesivir followed by a 14-day course of baricitinib, concluding that baricitinib plus remdesivir was superior to remdesivir alone in reducing recovery time and accelerating improvement in clinical status, notably among patients receiving high-flow oxygen or non-invasive MV [2]. Following this data, the SAR-CoV-2 treatment protocol in hundreds of hospitals was changed to a 10-day course of IV remdesivir and dexamethasone and a 14-day course of oral baricitinib. However, the data regarding the side effects of this combination therapy are limited to case reports[3]. This case report aims to highlight transient leukopenia as a potentially life-threatening side effect of this combination therapy.
CASE DESCRIPTION
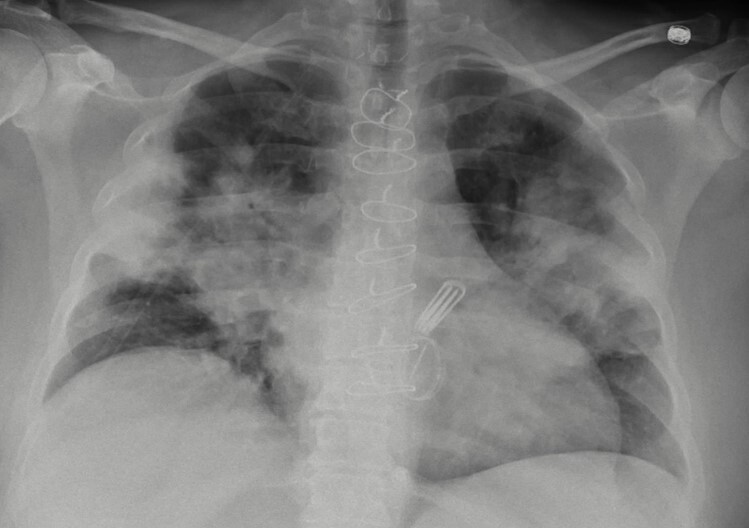
A 50-year-old woman with a medical history of a previous SARS-CoV-2 infection, cerebrovascular stroke with no residual neurological defects, coronary artery disease (CAD), mitral stenosis status post coronary artery bypass graft (CABG), and metallic mitral valve replacement, presented complaining of a 1-week history of fever, chills, haemoptysis and dyspnoea on exertion. On clinical examination, she was febrile at 39.2°C, tachycardic with a heart rate of 115 beats/minute, tachypnoeic with a respiratory rate of 30/minute, and hypoxaemic with oxygen saturation of 84% on room air with gradual improvement of her oxygen saturation after being placed on a 3-litre (l) nasal cannula. Her initial laboratory work-up showed a white blood cell (WBC) count of 11×103ml and positive SAR-CoV-2 infection on reverse-transcriptase polymerase chain reaction (RT-PCR). Chest x-ray showed bilateral perihilar pulmonary infiltrates reaching along the pleura bilaterally, sparing the upper and lower lung zones (Fig. 1).
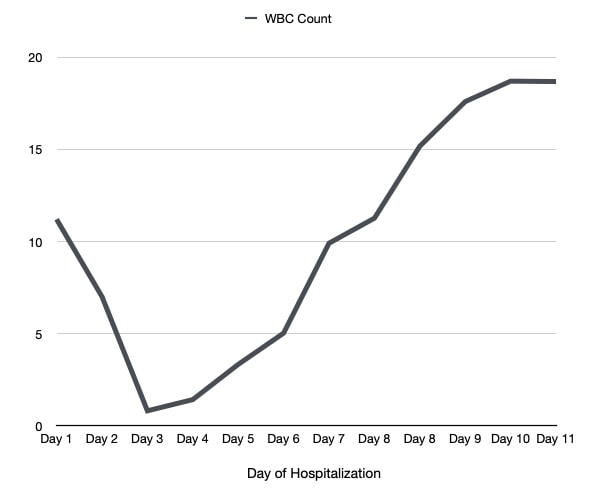
She was commenced on combination therapy for SAR-CoV-2: per oral (PO) 4 mg of baricitinib, intravenous (IV) 200 mg of remdesivir, and 8 mg of dexamethasone. On hospital day 2, she received a second dose of baricitinib, remdesivir and dexamethasone. Follow-up laboratory work-up revealed a WBC count of 7.0×103ml. On hospital day 3, the patient’s WBC count significantly dropped to 0.8×103ml, and remdesivir, baricitinib and dexamethasone were held. Further laboratory work-up demonstrated a mildly elevated erythrocyte sedimentation rate (ESR), C-reactive protein (CRP), liver function tests (alanine aminotransferase (ALT), and aspartate aminotransferase (AST)), D-dimer [and lactate dehydrogenase (LDH), while ferritin and rheumatological work-up was unremarkable. Peripheral blood smear showed normocytic/hypochromic anaemia and leukopenia. During the following days of hospital admission, her WBC count trended upward after the cessation of combination therapy, as illustrated in Fig. 2. Her symptoms also improved significantly, and she was weaned from oxygen and discharged to be followed on an outpatient basis.
Figure 1. Chest X-ray showing bilateral perihilar pulmonary infiltrates reaching along the pleura bilaterally, sparing the upper and lower lung zones
Figure 2. WBC count trend during hospital admission
DISCUSSION
Since the start of the SARS-CoV-2 pandemic, treatment modalities have been heavily researched. Although a definitive cure has not been established, treatment strategies are guided primarily by symptom severity. Studies such as ACTT-1 and ACTT-2 support the use of combination therapy with dexamethasone, remdesivir and baricitinib in patients who required hospitalization as it can help reduce the risk of severe illness and recovery time, and accelerate clinical improvement [4, 5]. Current National Institutes of Health (NIH) treatment guidelines recommend that for patients who require hospitalization without supplemental oxygen, remdesivir may be considered in those with a high risk of progression, while in patients requiring minimal supplemental oxygen, either remdesivir or dexamethasone monotherapy is recommended. Additionally, in patients requiring high-flow oxygen or non-invasive ventilation (NIV), either dexamethasone monotherapy or dexamethasone plus remdesivir is recommended. Baricitinib or IV tocilizumab can be added in patients requiring oxygen supplementation. For patients requiring MV or ECMO, dexamethasone plus IV tocilizumab is recommended if it can be given within 24 hours of admission [6].
We present a case of drug-induced acute transient leukopenia. There was a temporal relationship between medication exposure and the abrupt onset of acute leukopenia. Other causes of transient leukopenia such as viral infections, commonly infectious mononucleosis due to Epstein-Barr virus, or other viral infections such as cytomegalovirus, HIV and parvovirus B19, chronic bacterial infections, and inflammatory and autoimmune diseases (e.g., rheumatoid arthritis and sarcoidosis) were excluded [7].
The first evidence that combination therapy for SARS-CoV-2 can induce transient leukocytopenia was derived from a retrospective analysis that included 12 SARS-CoV-2 patients requiring supplemental oxygen in the form of a nasal cannula, high-flow nasal cannula or MV treated with combination therapy of dexamethasone, remdesivir and baricitinib. Leukocytopenia occurred within 24 hours in all 12 patients and resolved quickly even without medication discontinuation [3].
Although the mechanism of transient leukocytopenia after combination therapy is unknown, several mechanisms have been proposed. First, the temporally abnormal distribution of leukocytes might be a potential cause considering that leukopenia occurred shortly after exposure. Second, as baricitinib is a reversible inhibitor of Janus kinase (JAK) 1/JAK2, which suppresses cytokine-induced phosphorylation of signal transducer and activator of transcription 3 (STAT3), it potentially induces short-term leukopenia. Finally, inhibition of STAT3 phosphorylation reduces intracellular adhesion molecule 1 (ICAM1) expression, which might inhibit neutrophilic adhesion to blood vessel walls and facilitate extravascular migration [3].
CONCLUSIONS
Clinicians should be aware of transient leukopenia that can be induced with combination therapy with dexamethasone, remdesivir and baricitinib during the early phase of treatment of SARS-CoV-2 patients.