ABSTRACT
We report the case of a 31-year-old woman with a history suggestive of obstetric antiphospholipid syndrome (APS) with recurrent miscarriages, preterm labour and intrauterine fetal death. During her last pregnancy, she was referred to the Rheumatology Clinic at King Fahad Military Medical Complex, Dhahran, Saudi Arabia. Serology for connective tissue diseases and APS was negative on multiple occasions. During previous pregnancies, her obstetrician had initiated several trials of baby aspirin with and without prophylactic heparin, without success. We diagnosed her with seronegative obstetric APS (SN-APS). A specific regimen, consisting of combination therapy with baby aspirin, low-molecular-weight heparin, hydroxychloroquine (<5 mg/kg/day) and low-dose prednisolone, was attempted. She delivered a healthy baby even though it was born preterm at 30 weeks of gestation because of abruptio placentae. Obstetric SN-APS is rare and should be considered and, if the history is highly suggestive, treated similarly to seropositive obstetric APS to reduce mortality.
LEARNING POINTS
- Seronegative antiphospholipid syndrome (SN-APS) is very rare and often missed clinically.
- SN-APS should be treated similarly to seropositive obstetric APS to reduce recurrence.
- The antimalarial drug hydroxychloroquine should be considered 3 months before attempts at conception as it appears to decrease antiphospholipid levels.
KEYWORDS
APS, antiphospholipid syndrome, SN-APS, seronegative APS, IUFD, intrauterine fetal death
INTRODUCTION
Antiphospholipid syndrome (APS) is an autoimmune disorder that can be obstetric or vascular or present as a combination of vascular and obstetric symptoms. A number of studies have shown that in some cases, patients may present with clinical features of APS but with temporarily positive or persistently negative titres of antiphospholipid (APL) antibodies (Abs). For these patients, a definition of seronegative APS (SN-APS) has been proposed.
Seronegative APS is a challenging condition. Here we describe our own experience with a patient with a strong clinical suspicion of obstetric APS, with a history of recurrent pregnancy loss with nine miscarriages in the first trimester, preterm labour and intrauterine fetal death (IUFD), but whose serological results were negative for APS Abs. In this review, we summarize the evidence on SN-APS, its potential clinical relevance, and the antithrombotic therapeutic strategies available in this setting, as this condition is very rare and often missed clinically. Informed written consent was obtained from the patient for the publication of this case report.
CASE DESCRIPTION
A 31-year-old Saudi female patient with a medical history of glucose-6-phosphate dehydrogenase deficiency was referred to our rheumatology clinic from an obstetric clinic for further evaluation of recurrent pregnancy loss and to rule out possible APS. Nine of her miscarriages had occurred in the first trimester, and IUFD had occurred at 21 weeks of pregnancy. During previous pregnancies, her obstetrician had initiated several trials of baby aspirin with and without prophylactic low-molecular-weight heparin (LMWH), without success. The patient denied any history of connective tissue disease (CTD). There was no history of venous thrombus embolism and no family history of CTD.
Clinical findings
The patient was vitally stable, and her systemic examination was non-contributory. Her rheumatological examination results were not significant.
Diagnostic assessment
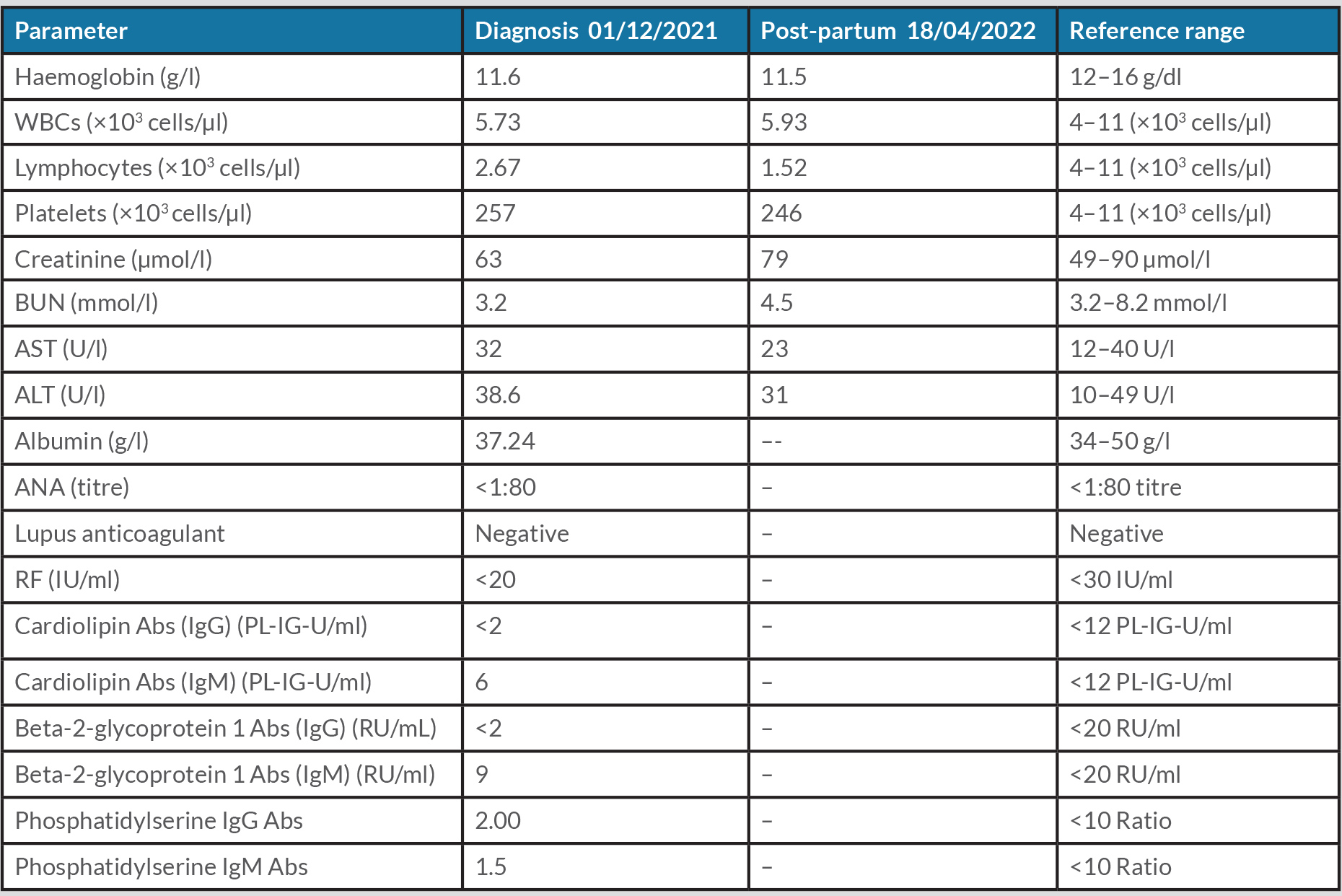
All of her blood tests on different previous occasions were negative for CTD and APS, including antinuclear antibodies (ANA), rheumatoid factor (RF), lupus anticoagulant (LAC), anti-cardiolipin (aCL) IgG and IgM Abs, beta-2-glycoprotein 1 (B2GP1) IgG and IgM Abs, phosphatidylserine IgG Abs, and phosphatidylserine IgM Abs (Table 1).
Table 1. Laboratory investigation results
Abs, antibodies; ALT, alanine aminotransferase; ANA, antinuclear antibodies; AST, aspartate aminotransferase; BUN, blood urea nitrogen; RF, rheumatoid factor; WBC, white blood cells.
Therapeutic intervention
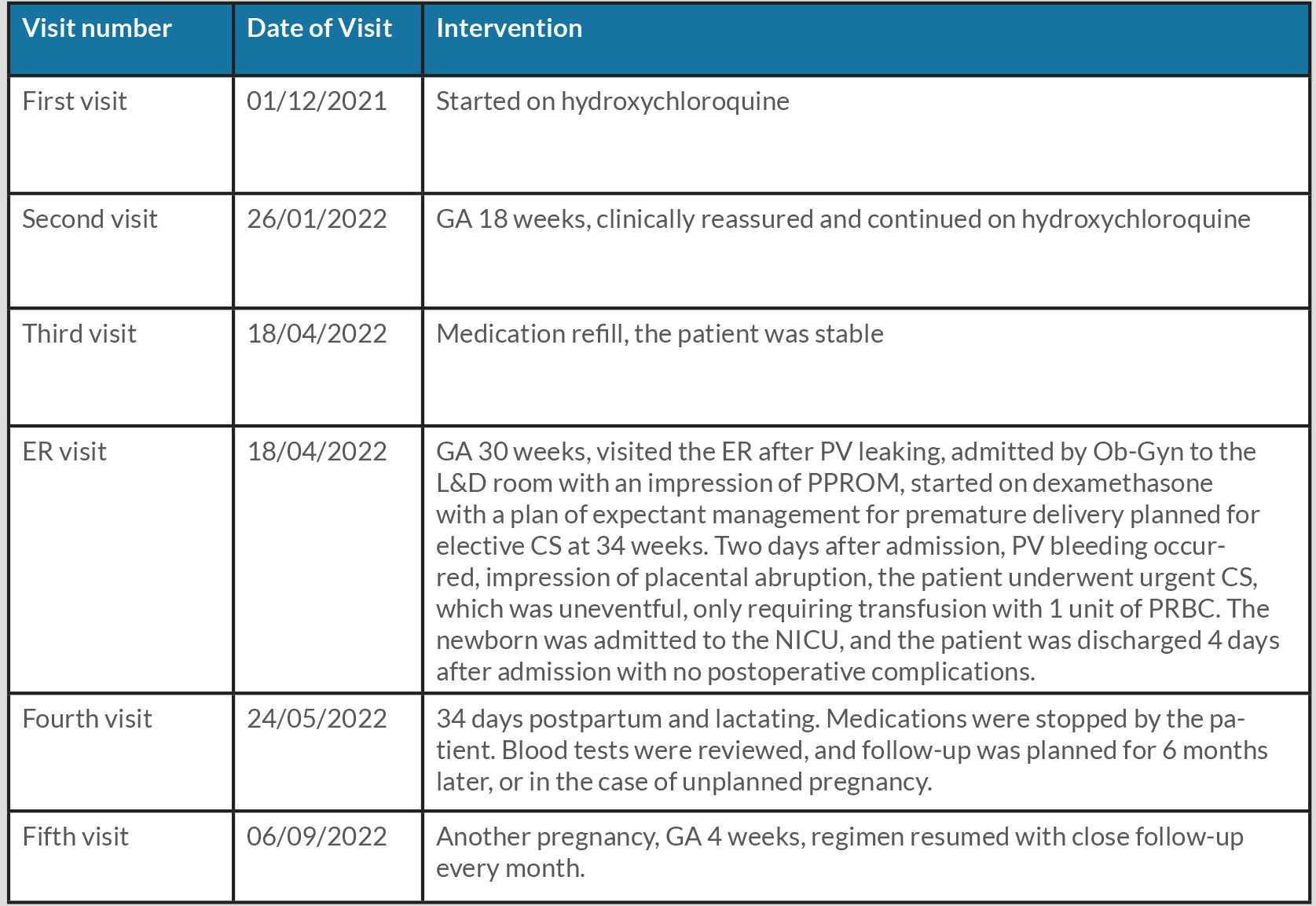
Prior to our evaluation, the patient had been prescribed aspirin 100 mg by mouth once daily, Clexane 40 mg subcutaneously twice daily, prednisolone 5 mg by mouth once daily, and folic acid 5 mg by mouth once daily by her obstetrician. At her first clinical visit with us, hydroxychloroquine was initiated at a dose of 400 mg by mouth once daily (<5 mg/kg/day), and the patient was closely followed up during her pregnancy until she visited the emergency room at gestational age (GA) 30 weeks because of vaginal leakage. She was admitted by the obstetrician-gynaecologist to the labour and delivery room with an impression of premature preterm rupture of the membranes (PPROM). She was started on dexamethasone with a plan of expectant management for premature delivery and planned for elective C-section (CS) at 34 weeks. Two days after admission, vaginal bleeding occurred, suggesting placental abruption. The patient underwent an urgent CS, which was uneventful and a transfusion of only 1 unit of packed red blood cells was required. The newborn baby was admitted to the neonatal intensive care unit, and the patient was discharged 4 days after admission with no postoperative complications after finally delivering a healthy preterm baby. She was followed up at 34 days after delivery with a healthy breastfed neonate. Four months after delivery, she was found to be pregnant at GA 4 weeks; the APS treatment regimen was resumed with close follow-up every month. A summary of the patient’s history and timeline of the outcomes are given in Table 2.
Table 2. Timeline
CS, C-section; ER, emergency room; GA, gestational age; L&D, labour and delivery; NICU, neonatal intensive care unit; Ob-Gyn, obstetrician-gynaecologist; PPROM, preterm premature rupture of membranes; PRBC, packed red blood cells; PV, per vagina.
DISCUSSION
APS is a systemic autoimmune disease characterized by thrombosis and/or pregnancy morbidity in the presence of persistently positive APL Abs [1]. Individuals who present clinical features highly suggestive of APS, but who are persistently negative for the APL Abs criteria, have led physicians to refer to these cases as ‘seronegative’ APS (SN-APS). Hughes and Khamashta first defined SN-APS in 2003 as clinical manifestations highly suggestive of APS in the absence of laboratory criteria, such as LAC, aCL and B2GP1 Abs [2]. The most commonly studied Abs are those against phosphatidylethanolamine, observed to be associated with blocking of the protein C pathway [3], phosphatidic acid and phosphatidylserine, a phospholipid that has a very similar structure to cardiolipin, yet differs with the addition of serine in place of the second glycerol group found in cardiolipin phosphatidylinositol, vimentin/cardiolipin complex, and annexin A2 and 5. Moreover, as many as 30 different non-criteria autoantibodies directed against phospholipids, glycoproteins and coagulation factors have been reported to be positive [4] and potentially linked to thrombotic risk in APS. However, assays to measure the levels of these Abs have not yet been standardized. The diagnosis of SN-APS is usually made by exclusion, but its recognition is very important so the most appropriate antithrombotic strategy can be initiated in order to reduce the rate of recurrence. SN-APS should be suspected in patients with a clinical history suggestive of APS, such as those with recurrent arterial venous thrombotic events, recurrent miscarriage, unexplained thrombocytopenia, persistent negativity of APL tested on at least two occasions, and when other causes of thrombosis have been excluded.
As noted in a study of SN-APS diagnosis based on non-criteria Abs published in 2020 [5], SN-APS diagnoses should be made after exclusion of other causes of inherited and acquired thrombophilic conditions. Moreover, routine testing for non-criteria Abs should only be considered in patients with high clinical suspicion of APS, such as our patient, who had recurrent pregnancy-related complications and responded well after the initiation of hydroxychloroquine and antithrombotic therapy.
The therapeutic intervention for SN-APS is similar to that for seropositive APS, as recommended in the EULAR Guidelines [6] for the management of obstetric APS. In women with obstetric APS but without thrombosis or pregnancy complications, treatment with low-dose aspirin (LDA) (75–100 mg/day) should be considered; in the presence of pregnancy complications, LMWH prophylactic doses during pregnancy are recommended in addition to LDA. Moreover, if the patient is on a combination of LDA and a prophylactic LMWH dose and continues to have pregnancy complications, the most common practice is to increase the amount of LMWH to a therapeutic dose. Although there is no supporting evidence, alternative therapeutic options include the addition of low-dose prednisolone or hydroxychloroquine, based on two observational studies with limited samples [7, 8]. In one of these studies, which included 30 patients with APS and obstetric complications, the addition of hydroxychloroquine decreased the rate of pregnancy loss from 81% to 19% (p<0.05) [8]. This result is consistent with the outcome observed in our patient, who continued to have obstetric complications despite being on LDA and LMWH combination therapy but delivered a preterm healthy baby at 30 weeks with uneventful postnatal care after the addition of hydroxychloroquine and low-dose prednisolone. The antimalarial drug hydroxychloroquine appears to decrease APL levels based on retrospective data, suggesting that hydroxychloroquine prophylaxis may have some benefits in patients with APS by reducing the risk of preeclampsia and preterm birth. As it takes around 3 months for hydroxychloroquine to have an effect, its administration should be considered before attempts at conception in patients suspected of having SN-APS [9].
CONCLUSION
Seronegative APS is a rare and challenging condition which, if missed, can lead to delay in diagnosis and management. Our study was limited by the scarcity of diagnostic tests as well as a lack of standardized clinical guidelines for the treatment of SN-APS. Further research is essential for standardizing management strategies for the early prevention of obstetric and non-obstetric complications.