ABSTRACT
Patients with symptomatic or malignant anomalous aortic origin of the right coronary artery (AAORCA) warrant surgical treatment to decrease morbidity and mortality. Various surgical techniques have been implemented including unroofing, reimplantation and bypass grafting. A 43-year-old woman presented with intermittent chest pain due to malignant AAORCA and received saphenous bypass grafting, instead of reimplantation, due to intraoperative spasm.
LEARNING POINTS
- Various surgical methods are available for the management of anomalous aortic origin of the right coronary artery (AAORCA), preferably unroofing when the intramural segment can be identified.
- Hypoplasia of the proximal segment, an acute take-off angle, and close proximity to the intercoronary pillar or commissure are limitations to unroofing, and alternative approaches are more appropriate.
- Coronary artery bypass graft, with either arterial or venous graft, can be performed when unroofing and reimplantation are not feasible. Measuring the distal anastomosis flow may help with a decision regarding native coronary artery ligation. It remains undetermined whether arterial or venous grafts provide superior outcomes.
KEYWORDS
Anomalous aortic origin of the right coronary artery, AAORCA, unroofing, coronary artery bypass graft, reimplantation
INTRODUCTION
Anomalous aortic origin of the right coronary artery (AAORCA) is an uncommon congenital malformation with varying clinical presentations ranging from asymptomatic to sudden cardiac death (SCD) [1]. Those with symptoms or high-risk lesions are usually managed by unroofing or reimplantation, rather than with coronary artery bypass graft (CABG), to avoid late attrition of bypass conduits [2]. Here, we present a case of AAORCA treated with a saphenous graft following intraoperative spasm of the RCA. The literature on the outcomes of those treated with bypass grafting and reimplantation is reviewed.
CASE DESCRIPTION
A 43-year-old female patient with obesity and hypertension presented with a 1-year history of intermittent exertional substernal pressure radiating to left shoulder and back. The pain, which lasted about 5 minutes per episode, had been more frequent and intense over the past week.
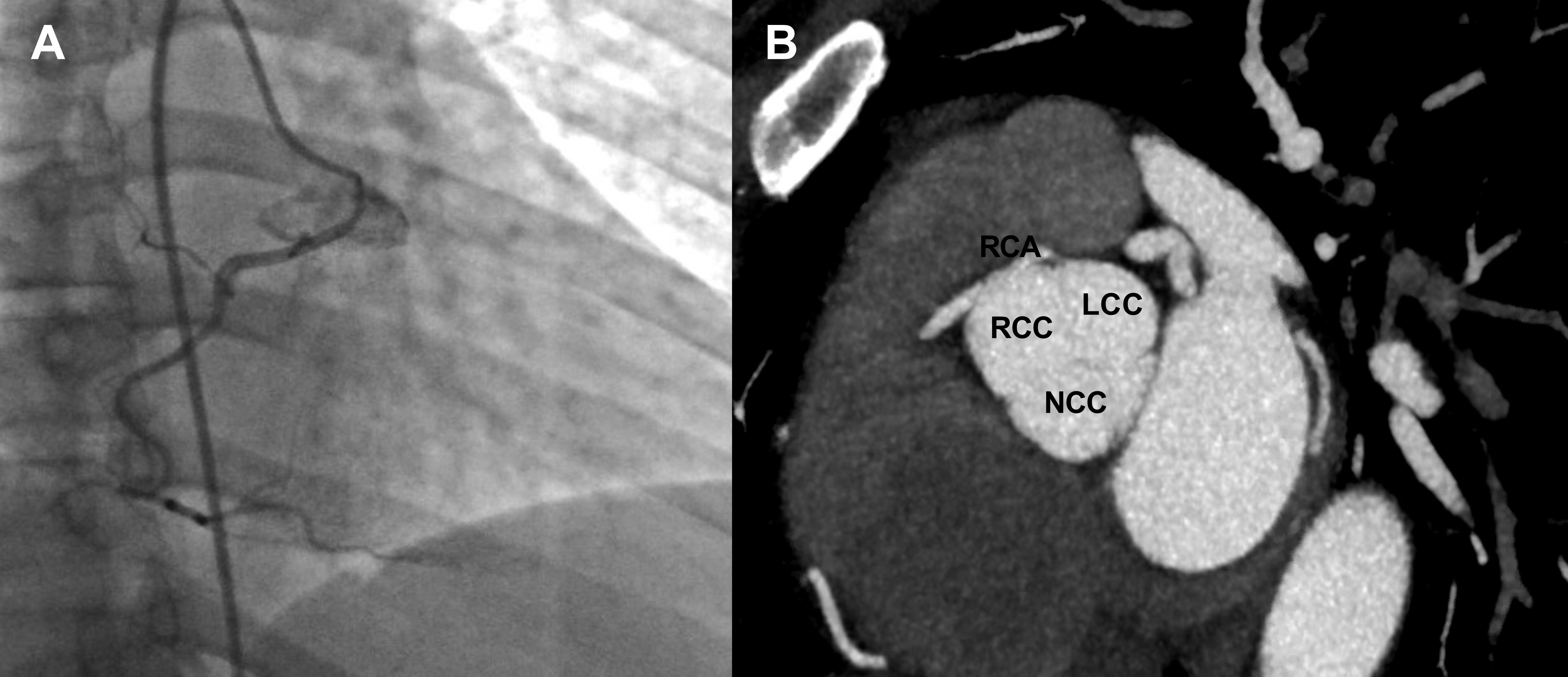
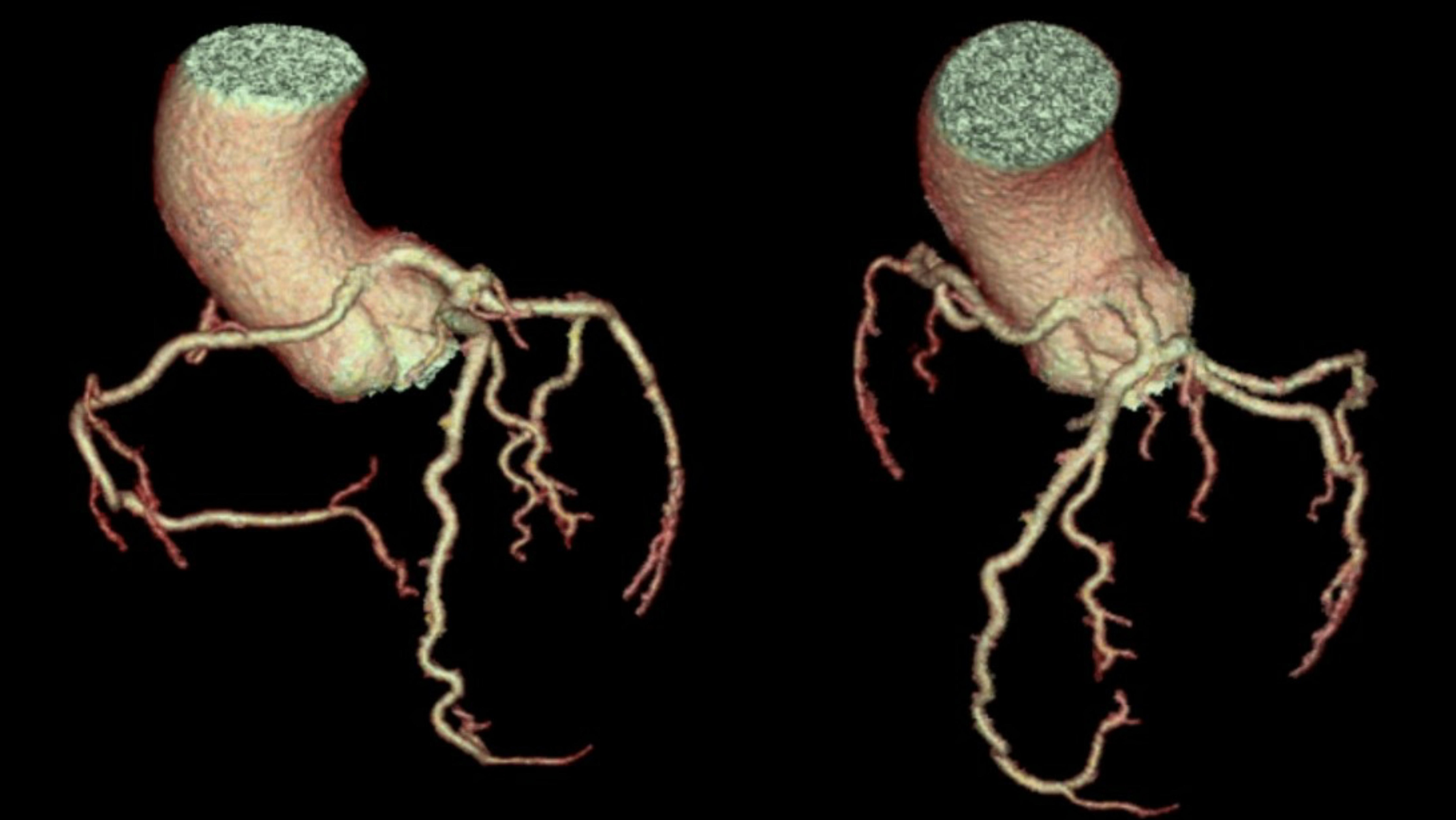
Upon initial evaluation, she was hypertensive at 175/110 mmHg and bradycardic at 50 bpm. Blood work was unremarkable, including negative serial troponin levels. Electrocardiography showed sinus bradycardia without ischaemic changes. Anomalous origin of RCA was incidentally discovered from computed tomography (CT) of the chest. Coronary angiography and ascending aortography were then performed, which revealed a patent RCA originating from the left coronary cusp (Fig. 1A). CT heart angiography with reconstruction revealed the RCA ostium in the left coronary cusp with a malignant course between the aorta and pulmonary artery and a slit-like orifice, without an obvious intramural segment (Figs. 1B and 2).
Figure 1. (A) Coronary angiography showing a patent RCA originating from the LCC. (B) Computed tomography angiography showed the RCA originating from the LCC with an inter-arterial course, a slit-like orifice, and minimal intramural segment. LCC, left coronary cusp; RCA, right coronary artery
Figure 2. Computed tomography three-dimensional reconstruction showing both the left main and right coronary arteries originating from the left coronary cusp
The base of the aorta was opened to locate the anomalous RCA, which was then dissected back to its origin near the left main coronary artery; however, unroofing was not performed due to its proximity to the aortic commissure. Reimplantation of the RCA was attempted. Despite extreme precautions, the vessel spasmed, rendering it deficient in length for a tension-free anastomosis. A saphenous vein graft was used instead. The postoperative course was uncomplicated.
DISCUSSION
Anomalous aortic origin of a coronary artery (AAOCA) has an estimated prevalence of 0.1%–0.7% with AAORCA being 3–6 times more common, but associated with less morbidity, than anomalous aortic origin of the left main coronary artery (AAOLCA) [3]. This condition is the second leading cause of SCD in healthy young adults [4], and approximately 17% of SCD in competitive athletes is also attributed to AAOCA [3].
The pathological mechanisms responsible for symptomatic AAORCA were previously thought to be a result of extrinsic compression of the inter-arterial section traversing between the aorta and pulmonary artery. However, high-risk anatomical features such as a restricted slit-like coronary orifice and a lengthy intramural segment subjected to compression within the aortic tunica media are now widely considered as the major contributors [5].
Most cases of AAOCA are diagnosed incidentally on echocardiography or CT scan. Cardiac magnetic resonance imaging or coronary CT angiography allows better spatial resolution and visualization of the anatomy [3]. For general practitioners, the important question is: ‘What is the next step in management?’. Expert consensus suggests assessing the ischaemic burden with an exercise stress test in combination with nuclear perfusion imaging or stress echocardiography [3]. It is recommended that surgery should be offered (a) to those with AAOCA with ischaemic chest pain, syncope or a history of SCD and (b) to those with asymptomatic AAOLCA arising from the right sinus of Valsalva with an inter-arterial course [3]. A decision to recommend surgery in asymptomatic AAORCA remains controversial; however, it should be considered especially in those with high-risk features [5].
Over the past decade, various case reports and studies focusing on the outcomes of each surgical technique have been published.
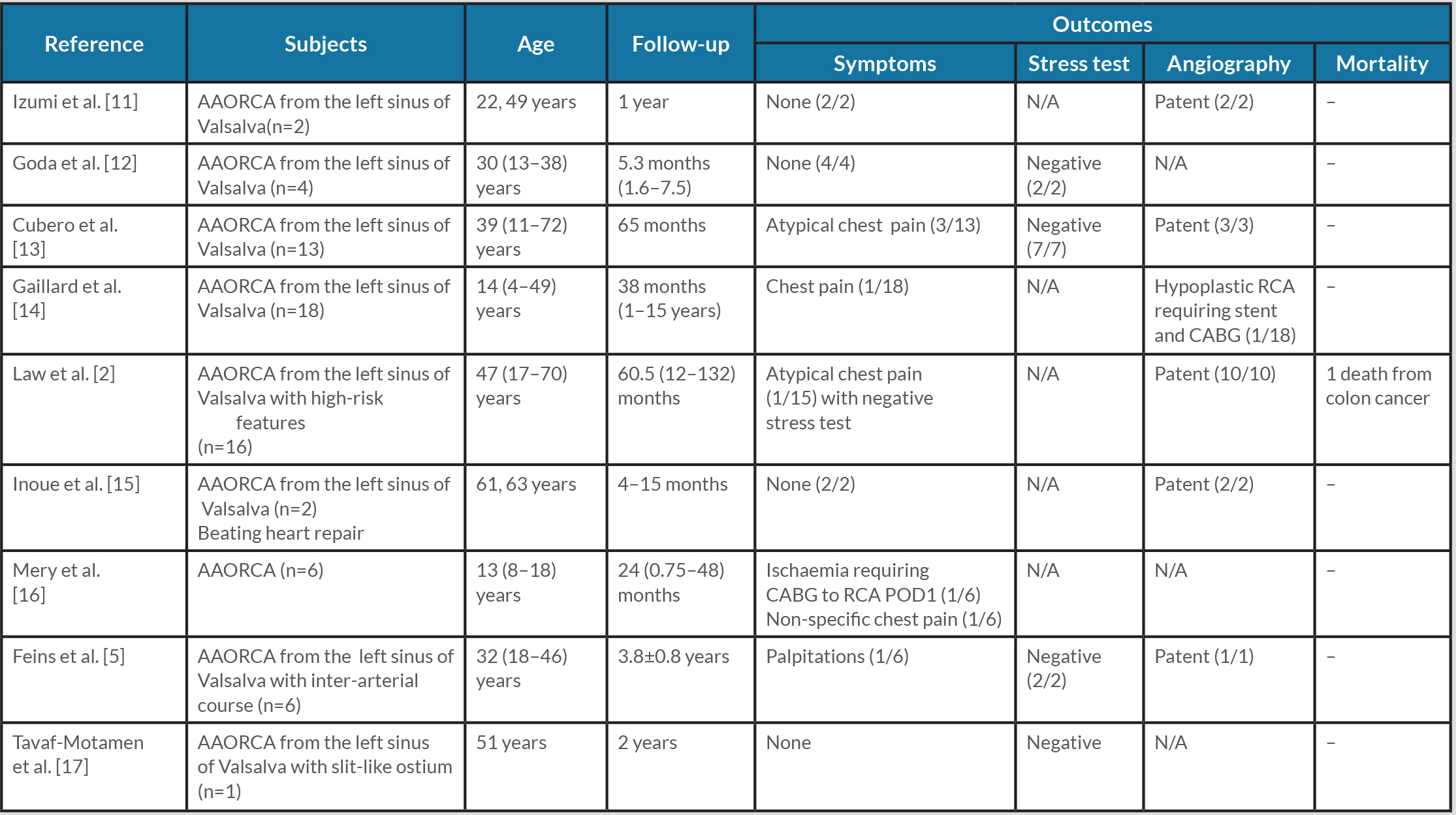
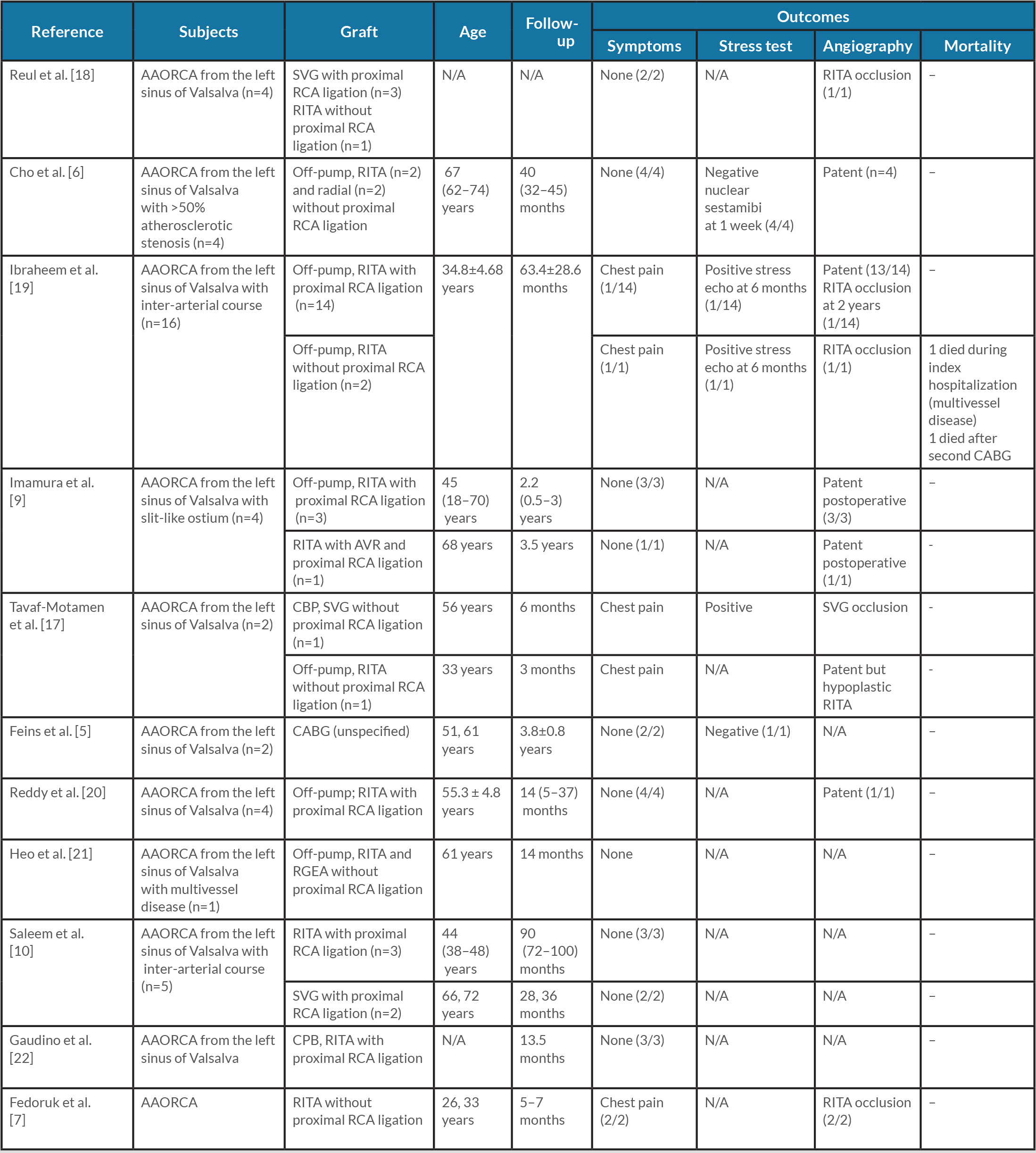
Unroofing may not be suitable if there is an inadequate intramural segment, hypoplasia of the proximal segment, an acute take-off angle, or close proximity to the intercoronary pillar or commissure, in which case alternative procedures are warranted [2, 4]. Available reports of cases treated with reimplantation or CABG are summarized in Tables 1 and 2.
Despite CABG being the most familiar procedure performed by cardiothoracic surgeons, concerns have been raised regarding graft failure due to competitive flow [6]. Previous case reports revealed the possibility of early graft failure when the internal thoracic artery (ITA) was used without proximal RCA ligation. Nevertheless, routine native artery ligation might not be the appropriate answer as initial flow from the ITA graft might be inadequate and catastrophic events may occur with graft occlusion [6, 7]. A study comparing proximal coronary stenosis and ITA graft patency, conducted by Sabik III et al., revealed that ITA graft patency is mildly decreased as competitive flow from the native artery increases, which is likely explained by vasoconstriction and disuse atrophy [8]. Cho et al. demonstrated favourable outcomes after a follow-up period of more than 3 years in four patients with AAORCA and concomitant RCA atherosclerotic stenosis (>50%) treated with either ITA or radial artery graft without proximal RCA ligation [6], suggesting that CABG with ITA graft might be a feasible option especially in elderly patients with coronary artery disease.
To addresses a concern regarding competitive flow, measuring flow distal to the anastomosis may be helpful. Three patients with a clean native artery treated with a right ITA graft and proximal RCA ligation remained asymptomatic after a mean of 2.2 years. A decision to perform ligation was made after increased graft flow during clamping was confirmed by transit-time flow measurement [9]. Nevertheless, justification for CABG in young patients without atherosclerosis remains debatable due to a concern about long-term graft patency. Also, the question as to whether venous or arterial grafts provide superior outcomes still needs clarification. Although saphenous vein grafts exhibit higher resting blood flow, ITA is known to have better long-term efficacy [10]. As this anomaly is relatively uncommon, it may be difficult to conduct larger studies to directly compare each graft type and surgical technique.
Table 1 .Outcomes of reimplantation for AAORCA
AAORCA, anomalous aortic origin of the right coronary artery; CABG, coronary artery bypass graft; N/A, not applicable; POD1, post-operative day 1; RCA, right coronary artery.
Table 2. Outcomes of CABG for AAORCA
AAORCA, anomalous aortic origin of the right coronary artery; AVR, aortic valve replacement; CABG, coronary artery bypass graft; CPB, cardiopulmonary bypass; N/A, not applicable; RCA, right coronary artery; RGEA, right gastroepiploic artery; RITA, right internal thoracic artery; SVG, saphenous vein graft.